A recent review has addressed critical issues within the Central Secretariat Stenographers’ Service (CSSS), aiming to foster a more efficient, responsive, and career-centric environment for its members. The third cadre restructuring committee identified significant challenges, including a skewed cadre structure where entry-level Stenographer Grade ‘D’ positions were disproportionately fewer than Personal Assistant (PA) roles, leading to numerous unfilled vacancies and stagnation. This imbalance, coupled with outdated staffing norms and the impact of evolving IT environments, necessitated a comprehensive overhaul.
The restructuring introduces several key changes. The nomenclature for Stenographer Grade ‘D’ will be simplified to ‘Stenographer’. Significant adjustments to cadre strength are proposed: an increase in Stenographer Grade ‘D’ positions from 1324 to 1908, a decrease in PA posts from 2627 to 1900, while Private Secretary (PS) roles remain at 2090. Higher-level positions like Principal Private Secretary (PPS) and Senior Principal Private Secretary (Sr. PPS) will see an increase from 780 to 855 and 143 to 211, respectively, to address stagnation in feeder grades. The overall strength of the cadre will remain consistent.
To improve career progression and fill vacancies, recruitment rules for PA and PS grades will be revised. A 75% Seniority Quota (SQ) and 25% Limited Departmental Competitive Examination (LDCE) split for filling vacancies is recommended, with no re-introduction of direct recruitment at the PA level. Deserving Stenographer Grade ‘D’ officials, after 6 years of approved service and with a graduation degree, will gain an additional promotional avenue by being permitted to appear in the LDCE for entry into the Assistant Grade of the Central Secretariat Service (CSS).
Capacity building is a major focus, with recommendations for posting Stenographers Grade ‘D’ in various sections to develop multi-skilling, essential for future roles. Bilingual training is suggested for all cadre officials, ideally within their first two years, and Stenographers Grade ‘D’ with 12th-grade qualification will be encouraged to pursue graduation through correspondence courses to enhance their career prospects. Additionally, the Department of Personnel and Training (DoPT) is tasked with revising grade-wise duties and responsibilities to align with current functional requirements. An 8% reserve is also recommended at the Stenographer Grade ‘D’ level to effectively manage manpower across ministries.
These strategic changes are anticipated to not only enhance the effectiveness of the CSSS but also yield substantial financial savings, projected at over Rs. 1.28 crore per month, reinforcing a more sustainable and equitable service structure.
SOURCE PDF LINK :
Click to access CadreRestructuringReport.pdf
Click to view full document content
No.15/1/2014-CS.II-(C)
Government of India
Ministry of Personnel, Public Grievances & Pensions
Department of Personnel & Training
3rd floor, Lok Nayak Bhavan,
Khan Market, New Delhi-100 003
Dated: the 16th April, 2015
Circular
Sub: Cadre Review of Central Secretariat Stenographers’ Service (CSSS) — regarding.
3rd Cadre Restructuring Committee (CRC) constituted to review the service conditions of the CSSS personnel has submitted its report. The report of the Committee along with its major recommendations and duties of CSSS personnel are attached herewith for information.
- Accordingly, all stakeholders viz. Ministries/Departments, Service Associations and individual officers may submit their comments on the report latest by 15th May, 2015. The comments may also be furnished via e-mail at kameshwar.mishra@nic.in
Kameshwar Mishra
Under Secretary to the Govt. of India
Telefax: 24623157
To
All Ministries/Departments
Service Associations and individual officers
REPORT OF THE COMMITTEE
ON
$3^{\text {R }}$ CADRE RESTRUCTURING OF CSSS
PREFACE
A Committee for the Cadre Restructuring (3rd) of the Central Secretariat Stenographers’ Service (CSSS) was constituted vide order dated 29.12.2014, which was modified vide order dated 06.02.2015, with a specific mandate to review the structure of the CSSS so as to harmonize the functional needs with the legitimate career expectations of its members and to suggest measures to enhance the effectiveness of the Service.
2. The Committee approached the issue in a holistic manner and arrived at its recommendations keeping the larger public interest in view. We hope that the suggested measures would enhance the effectiveness of the Service and usher in better cadre management of the Central Secretariat Stenographers’ Service in future.
Vandana Shame.
(Vandana Sharma)
Director, CS-II
Member Secretary
Archana Varma
(Archana Varma)
Joint Secretary (AT\&A)
Member
(Annie George Mathew)
Joint Secretary (Pers)
Member

(T. Jacob)
Additional Secretary(S\&V)
Chairman
New Delhi, 27th February, 2015
.
Executive Summary
Third Cadre Review of the Central Secretariat Stenographers’ Service
Central Secretariat Stenographers’ Service (CSSS) is one of the three services in the Central Secretariat which was constituted to provide secretarial and office support to officers in the Secretariat and its attached offices. The service conditions of the members of the service are at present regulated through CSSS Rules, 2010 and Regulations made thereunder. The service has so far had two cadre reviews and has undergone substantial changes in the intra grade cadre strength.
ii. The Committee had interactive sessions with the Associations of the CSSS, individual officers as well as some cadre units. The Committee met on several occasions to deliberate and examine the issues before it. Before arriving at its recommendations, the Committee took into account the views and grievances of the CSSS Associations, functional requirement in the Government, career expectations, availability of manpower and other factors relevant in the matter. The Committee was of considered view that the cadre structure of CSSS should be such as to enable it to attract best talent in the service and provides the members of the service with excellent possible career advancement opportunities which, in turn, encourages competence and innovation, ensures capacity building at all level and makes the service more efficient, effective and responsive.
iii. The Committee noted that the existing cadre structure of the service is skewed inasmuch as the sanctioned strength at the Stenographer Grade ‘D’, which is the entry grade, is almost half of the strength at the Personal Assistant (PA) grade which has resulted in unfilled vacancies at PA level.
iv. It was further noted that the existing scale of prescribed CSSS staff attached with the officers is not realistic keeping in view the functional requirement.
v. The Committee recommended restructuring of CSSS cadre to make it sustainable in the long run.
vi. The Committee recommended increase in the number of posts at Steno Grade ‘D’ level keeping in view the fact that the Executive Assistant Scheme has still not been finalized.
vii. The Committee noted that the posts at Personal Assistant level (PA) needs to be reduced to correct the cadre structure. As the intake through LDCE mode at PA level has been considerably low in comparison to the existing vacancies, the Recruitment Rules need to be amended to increase the intake through Seniority Quota (SQ) mode and decrease the intake through LDCE mode.
viii. The Committee also suggested additional promotional avenues for deserving CSSS officials by recommending that Stenographers Grade ‘D’ with 6 years of approved service may also be permitted to appear in the LDCE for entry into Assistant grade of CSS.
ix. The Committee noted that the capacity building of the cadre has been suitably addressed under the existing Cadre Training Plan (CTP) which is mandatory for further promotion. The Committee recommended that the officials in the cadre may be trained bilingually keeping in view the job requirement.
x. The Committee observed that in pursuance of the recommendations of the $14^{\text {th }}$ Finance Commission, certain Ministries/Departments in the Central Government dealing with State Governments are likely to be downsized. Therefore, there is a need to devise a cadre structure which strikes a balance between the functional requirements in the Government as well as the legitimate career expectations of the members of the service.
xi. The Committee proposed revised norms for scale of CSSS personnel attached with the officers at various levels.
xii. The Committee proposed that the nomenclature of Stenographer Gr. ‘D’ be changed to Stenographer. It has further been recommended that the strength of Stenographers Grade ‘D’ be increased from the existing number of 1324 to 1908.
xiii. The sanctioned strength of Personal Assistant (PA) may be decreased from 2627 to 1900. The Recruitment Rules for the PA grade may also be revised to provide for filling up of $75 \%$ vacancies through Seniority Quota (SQ) and $25 \%$ of the vacancies through Limited Departmental Competitive Examination (LDCE).
xiv. The existing sanctioned strength of 2090 of Private Secretary (PS) may be kept unchanged.
xv. The sanctioned strength of Principal Private Secretary (PPS) may be enhanced from the existing strength of 780 to 855 .
xvi. The sanctioned strength of Senior Principal Private Secretary (Sr. PPS) may be increased from the existing strength of 143 to 211.
xvii. The Committee has not found functional justification for creation of a post in the CSSS hierarchy at the level of Joint Secretary.
xviii. The Committee noted the need to increase the promotional avenues of deserving CSSS officials. It has, therefore, been recommended that Stenographers Grade ‘D’ with 6 years of approved service may also be permitted to appear in the LDCE for entry into Assistant grade of CSS.
xix. The Committee is of the view that, keeping in view the promotional prospects of Stenographers Grade ‘D’, there is no need to introduce the Direct Recruitment component at PA level for entry into CSSS.
xx. The Committee has recommended that the duties \& responsibilities of the CSSS personnel may be revised grade wise by the concerned Administrative Division in Department of Personnel \& Training keeping in view the functional requirement.
xxi. The Committee has recommended that a reserve of $8 \%$ of the revised cadre strength of PA and above levels in CSSS should be operated at Stenographers Grade ‘D’ level to meet the shortfall and requirement of manpower across Ministries/Departments.
xxii. Residency period for promotion to the next grade was also reviewed by the Committee. It has been recommended that existing residency period in a grade is appropriate and should, therefore, be retained.
CHAPTER – I
Introduction, Composition, ToR and Methodology
Central Secretariat Stenographers’ Service (CSSS) is one of the three services in the Central Secretariat, the other two being Central Secretariat Service (CSS) and Central Secretariat Clerical Service (CSCS). The CSSS was constituted to provide secretarial and office support to officers in the Secretariat and its attached offices. The service conditions of the members of the service were earlier regulated through CSSS Rules, 1969 and Regulations made thereunder. The earlier rules were repealed and presently the service is regulated through CSSS Rules, 2010 and Regulations made thereunder.
2. The Government constituted a ‘Group of officers’ on cadre structure of CSSS in October, 2003. The Group submitted its report in 2004. The recommendation of the Group of officers on cadre structure of CSSS were considered by the Government and several decisions were taken with the approval of the Cabinet in 2005. The second cadre restructuring was undertaken in 2010 and the recommendations were implemented by issuing orders in 2011. Subsequently, various issues relating to the service matters of CSSS cropped up and it was decided by Minster of State (Public Grievances and Pensions) that the third cadre review of CSSS be undertaken forthwith to address all the relevant issues. Government, vide Department of Personnel \& Training Order No. 15/1/2014-CS.II (A) dated 29.12.2014, constituted the Cadre Restructuring Committee, hereinafter referred to as the ‘Committee’.
The composition of the Committee and the Terms of Reference (ToR) are annexed as Annexure I. Briefly, the ToR read as under:
(i) To review the structure of CSSS cadre with a view to harmonise the functional requirements with the career expectation of its members;
(ii) To assess the magnitude of stagnation in various grades of CSSS and suggest remedial measures both short term and long term so as to reduce promotion blocks and at the same time prevent gaps from building up;
(iii) To suggest measures to enhance the effectiveness of the service and capacity building of its members.
(iv) To take into view the suggestions of stake holders;
(v) To review the entitlement for stenographic assistance to various category of officers of Government of India;
(vi) To examine any issue as referred to it by the cadre controlling authority.
Subsequently the composition of the Committee was partially modified vide OM dated $6^{\text {th }}$ February, 2015 (Annexure II).
1st Cadre Review of CSSS-2003
- The cadre review of CSSS was undertaken for the first time in the year 2003. At that time there were five grades in the service viz. Stenographers Grade D, Stenographers Grade C (PA), Private Secretary (PS), Principal Private Secretary (PPS) and Sr. Principal Private Secretary (Sr.PPS) The grades of Steno Grade D, PA and PS were decentralized in 33 cadres. The cadre was centralized from PPS onwards.
- The important decisions on account of First Cadre Restructuring of CSSS were as under:-
(i) Upgradation of posts at the level of PAs and PSs.
(ii) Stoppage of direct recruitment in the PA grade
(iii) Raising of minimum educational qualification to $12^{\text {th }}$ standard for direct recruitment to Steno Gr. D and introduction of the element of computer literacy.
(iv) The mode of recruitment of PA was changed by way of $50 \%$ through LDCE and $50 \%$ through seniority from Steno Grade D. Element of computer literacy was also introduced in the LDCE for promotion to PA.
(v) The cadre strength of CSSS in respect of various grades was fixed as under:-
| Sl. No. |
Grade (Designation) | Strength |
|---|---|---|
| 1 | Sr. PPS | 68 |
| 2 | PPS | 130 |
| 3 | PS | 1650 |
| 4 | PA | 2793 |
| 5 | Steno Grade D | 1958 |
| 6 | Total | $\mathbf{6 5 9 9}$ |
- The following changes were subsequently made in cadre structure of CSSS :-
a. The number of posts in the grade of PS was reduced to 1598 from 1650 and the number of PPS was increased to 182 from 130 vide O.M. dated 18.11.2005.
b. The grade of PSO (NFSG) in the pay scale of Rs. $37400-67000+$ G.P. of Rs. 8700/- was introduced vide O.M. dated 20.03.2009 in pursuance of the recommendation of the $6^{\text {th }}$ Central Pay Commission. $15 \%$ of the posts of Sr. PPS and PPS in CSSS are to be operated at the level of PSO.
c. 45 posts of PPS were upgraded to the level of Sr. PPS vide O.M. dated 06.04.2009.
$2^{\text {nd }}$ Cadre Restructuring
- The Second Cadre Review of CSSS was initiated in 2010 and the necessary orders for implementing the recommendations were issued in 2011. Broadly, it resulted in the following decisions:
(i) Creation of additional 25 posts of Senior Principal Private Secretary (Sr. PPS).
(ii) Creation of additional 625 posts Principal Private Secretary (PPS).
(iii) Up-gradation of 400 posts of Personal Assistant (PA) to Private Secretary (PS) grade.
(iv) Filling-up all resultant vacancies in the PS grade due to up gradation and creation of 25 posts in Sr. PPS grade and 625 posts in PPS grade by promotion, through Seniority Quota, as a onetime measure.
(v) filling-up all existing direct recruitment vacancies in the PA grade and fresh vacancies percolating to the PA grade, due to creation of 25 posts in Sr. PPS grade and 625 Posts in PPS grade, only by promotion, through Seniority Quota, as a onetime measure.
(vi) The cadre strength of various grades of CSSS was revised w.e.f. 20.01.2011 as under:-
| Sl. No. |
Grade (Designation) | Strength |
|---|---|---|
| 1 | PSO | |
| 2 | Sr.PPS | 140 |
| 3 | PPS | 773 |
| 4 | PS | 2041 |
| 5 | PA | 2524 |
| 6 | Steno Grade D | 1282 |
| Total | $\mathbf{6 7 6 0}$ |
(vii) The following table illustrates as to how the number of sanctioned posts in CSSS at all levels have increased after the first and second cadre restructuring:
Changes in cadre structure after 1st and 2nd cadre restructuring
| Sl. No. | Grades | Sanctioned strength after 1st CRC* | Sanctioned strength after the 2nd CRC** | No. of posts encadred since then | Existing Strength |
|---|---|---|---|---|---|
| 1. | PSO | Nil | 140 | 3 | 143 |
| 2. | Sr.PPS | 68 | 773 | 7 | 780 |
| 3. | PPS | 130 | 2041 | 49 | 2090 |
| 4. | PS | 1650 | 2524 | 103 | 2627 |
| 5. | PA | 2793 | 1282 | 42 | 1324 |
| 6. | Steno Grade D | 1958 | 6760 | 204 | 6964 |
- As per OM No. 10/3/2004-CS.II(Part III) dated 18.07.2005.
** As per OM No. 20/51/2009-CS.II(A)(Vol.II) dated 25.02.2011.
3rd Cadre Restructuring : Methodology Adopted
- The Committee sought written submissions from the concerned Associations of the CSSS as well as individual officers and also offered them the opportunity for presenting their view-points before the Committee. A list of Associations which met the Committee, made presentations before the Committee is placed at Annexure-III. The Committee held an interactive session on 10.02.2015 with the officers of some Ministries/Departments in this regard.
The Committee met on various occasions to deliberate and examine the issues referred to it. It took into consideration views and grievances of Associations, functional requirement of the government, availability of manpower and other relevant factors into consideration before arriving at its recommendations.
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Balance carbon atoms first.
- Then balance hydrogen atoms.
- Finally, balance oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
- Balance the equation:
- Balance oxygen atoms first.
- Then balance oxygen oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
Types of Chemical Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.015 \, \text{g/mol} = 18.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$ = Pressure (atm)
- $$V$$ = Volume (L)
- $$n$$ = Moles of gas
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁ = Pressure (atm)
- P₂ = Volume (L)
- P₃ = Moles of gas (L)
- $$P_1V_1 = P_2V_2$$
- Equation: $$P_2V_2 = P_3V_3$$
Charles’s Law
- Equation: $$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
- Variables:
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
- $$T_1$$ = Temperature (K)
- $$T_2$$ = Volume (L)
- $$T_3$$ = Temperature (K)
Boyle’s Law
- Equation: $$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
- Variables:
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
- $$T_1$$ = Temperature (K)
- $$T_2$$ = Volume (L)
- $$T_3$$ = Temperature (K)
Thermochemistry
Enthalpy Change (ΔH)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$ = Heat transferred at constant pressure.
- $$q_p$$ = Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – \Delta H_0$$
- Variables:
- $$\Delta H$$ = Heat transferred at constant pressure.
- $$\Delta H_0$$ = Heat transferred at constant pressure.
Hess’s Law 2.0
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – \Delta H_0$$
- Variables:
- $$\Delta H$$ = Heat transferred at constant pressure.
- $$\Delta H_0$$ = Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$ = Cell potential
- $$E^\circ$$ = Standard cell potential
- $$R$$ = Ideal gas constant
- $$T$$ = Temperature (atm)
- $$n$$ = Number of electrons transferred
- $$F$$ = Faraday constant
- $$Q$$ = Reaction quotient
CHAPTER – 2
Structure of the Service
Central Secretariat, at lower to middle levels, primarily consists of three services, namely, Central Secretariat Service (CSS), Central Secretariat Clerical Service (CSCS) and Central Secretariat Stenographers’ Service (CSSS). All the three services have their own respective Recruitment Rules and Regulations issued thereunder which govern the cadre management of each service.
2. The CSSS was constituted to provide secretarial and office support to the officers in the Secretariat and its attached offices. CSSS is a specialized service whose personnel have the technical core competency of stenographic skills. While undertaking restructuring of the Service following established principles, followed in respect of other services also, have to be kept in view :-
i The scale of pay for any post has a direct relation to the qualifications, mode of recruitment, responsibilities and duties attached to the post;
ii As the stenographers perform a skilled job, the level of efficiency in the skill has to be given due consideration in determining his suitability for posting with officers of different levels; and
iii The structure of the service has necessarily to be built around the functional requirements of the organization and the career aspirations of its members.
3. The net effect of two cadre reviews that have been done in the past has kept the strength of CSSS more or less the same. However, the grade wise structure has undergone a substantial change. After the second cadre review, the cadre structure of the CSSS has become skewed as the sanctioned post in PA grade is almost twice the total strength of Steno Grade D. The sanctioned strength of Stenographers grades D, which is the only entry grade into the service, is 1324 (at present) viz-a-viz the
present sanctioned strength of 2627 in the PA grade. This has resulted in huge number of unfilled vacancies in the PA grade. Unless steps are taken to correct this structure there will be a serious crisis of manpower crunch which is already being felt across Ministries/Department. Many of the Ministries/Departments have resorted to engaging outsourced staff besides hiring the services of retired officials as consultants. This is not a desirable situation keeping in view the continuity and quality of manpower. Efficient human resource management is integral to the overall efficiency of the working of the Central Government. Therefore, it is essential to devise a cadre structure which attracts best talent to this service and provides excellent career advancement opportunities, encourages competence and innovation, ensures capacity building at all levels and makes the service more efficient, effective and responsive.
4. The posting of CSSS officers with the officers in the Central Secretariat is being governed by the O.M. No. 10/3/2004-CS.II (Pt.VII) dated $28^{\text {th }}$ October, 2005 which is placed at Annexure-IV. It is observed that this order does not adequately address the staff requirement at senior level. During interaction with some Ministries/Departments it was highlighted that the actual staff posted at senior level was much beyond the norms which resulted in manpower crunch at the lower level. This issue needs to be resolved so that the entitlement norms are based on practical requirement.
5. The Committee also observed that several grades in higher scales have been prescribed in the CSSS without much change in work content in different grades. There is no specific job description prescribed for the grades of PPS/Sr. PPS/PSO. They all perform similar function and this leads to frustration amongst the officials and at the same time Government does not gain much from the experience of senior officials in this service.
- Over the last decade, there has been a sea-change in the work environment in Central Ministries/ Departments due to IT innovations. This has resulted in increase in efficiency and speed in performance of duties, including those by the CSSS personnel also.
- With increased working on computers, demand for stenographic skill in work places has declined resulting in decline in supply of skilled persons due to many training institutions closing down. This trend is likely to continue.
- The restructuring exercise of CSSS should look at the structure of the service which would be sustainable in the long term keeping in view the functional aspects, reporting hierarchy and reasonable career opportunities. For sustainable cadre structure of CSSS, there is a need to:-
(i) Increase the recruitment at Steno Grade D level.
(ii) Decrease the number of posts at PA level.
(iii) Introduce additional promotional avenues to meritorious officials.
(iv) Increase the number of posts at PPS and Sr. PPS level. - Keeping the above requirements in perspective, the Committee feels that there is a need to increase the number of posts at Steno Grade D level as this is the only feeder cadre of CSSS. It may be mentioned that the concept of e-office has been introduced in the Central Secretariat. This is likely to reduce dependence on personal staff for stenographic assistance in future. This would also necessitate a change in the organization and staffing in future. However, the recruitment at Steno Grade D level needs to continue till the Executive Assistant Scheme, which is under reference to the $7^{\text {th }}$ Pay Commission, is finalized. It is
further suggested that the recruitment of the increased posts may be phased out in 2 years to avoid bunching which may adversely affect the promotional opportunities for the new recruits.
10. The cadre structure has to be corrected which requires that the number of posts at PA level be reduced. As per the present recruitment rules $50 \%$ vacancies at PA grade are filled through SQ mode and $50 \%$ vacancies are filled through LDCE mode. Presently, there is a huge number of unfilled posts in this grade. This is due to lack of eligible officers in the feeder grade. Secondly almost negligible number of officials qualify the LDCE conducted by SSC. The reduction in posts in PA grade will not affect the promotional avenues for Steno Grade D and would also solve the problem of manpower crunch being faced by the cadre.
11. Presently $50 \%$ vacancies in PA grade are filled through LDCE mode. As per the details available negligible number of candidates have qualified LDCE in the last 4 years. This has resulted in a huge number of unfilled vacancies. It is, therefore, suggested that the percentage of vacancies to be filled through SQ mode be increased from the existing $50 \%$.
12. Presently, the CSSS officials can avail of the opportunity of entry into CSS at SO level through SO/PS grade LDCE conducted by UPSC. There is a need to increase the promotional avenues for deserving CSSS officials. An opening in this regard may be provided at Assistant level of CSS through LDCE mode. As per the existing recruitment rules $10 \%$ of the vacancies in Assistant grade of CSS are filled through eligible officials from CSCS. Stenographers Grade D with 6 years of approved service may also be permitted to appear, along with UDCs of CSCS, at the LDCE for entry into Assistant grade of CSS.
- With a view to enlarge the job profile and ensuring multi skilling of CSSS officials, Stenographers Grade D be also posted in sections. This would provide them the requisite experience which would help them in case they qualify the Assistant grade LDCE.
- Capacity building of the cadre has been addressed to under the existing Cadre Training Plan (CTP) which is mandatory for further promotion. The training modules of the CTP are reviewed and revised from time to time keeping in view the job requirement. Taking into account the official requirement, it is essential that the cadre is trained bilingually. In this regard it would be appropriate, if this training is given during the first two years to ensure a well trained and efficient cadre.
- The eligibility requirement of Steno grade D is $12^{\text {th }}$ standard. It is suggested that the Stenographers Gr. D be given an opportunity to complete their graduation through correspondence course through IGNOU or any other University/Institution to enable them to secure better career prospects. This would also provide a more competent and efficient work force to the Government.
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Balance carbon atoms first.
- Then balance hydrogen atoms.
- Finally, balance oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
- Balance the equation:
- Balance oxygen atoms first.
- Then balance oxygen oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
Types of Chemical Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 2 moles of $$H_2O$$.
- $$m = 2 \, \text{mol} \times 48.015 \, \text{g/mol} = 24.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$ = Pressure (atm)
- $$V$$ = Volume (L)
- $$n$$ = Moles of gas
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁ = Pressure (atm)
- P₂ = Volume (L)
- P₃ = Moles of gas
- $$P_1V_1 = P_2V_2$$
- Equation: $$P_2V_2 = P_3V_3$$
Charles’s Law
- Equation: $$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
- Variables:
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
Avogadro’s Law
- Equation: $$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
- Variables:
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Change in Enthalpy (ΔH): $$ΔH = q_p$$
- Change in Enthalpy (ΔH_2): $$ΔH_2H_2 + Q_p$$
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p \Delta H_2$$
- Equation: $$\Delta H_2H_2 \Delta H_2 \Delta H_2 \Delta H_2$$
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$ = Cell potential
- $$E^\circ$$ = Standard cell potential
- $$R$$ = Ideal gas constant
- $$T$$ = Temperature (atm)
- $$n$$ = Number of electrons transferred
- $$F$$ = Faraday constant
- $$Q$$ = Reaction quotient
CHAPTER – 3
The way forward:-
Keeping the existing scenario in view, the Committee has examined the following possible routes to ensure a better cadre structure which would increase the available manpower at the Central Secretariat for efficient, effective and responsive governance.
I. Entitlement norms:
The Committee during its interaction with Associations and representatives of the Ministries found that the actual staff posted at senior level was much beyond the norms laid down vide OM dated $28^{\text {th }}$ October, 2005. The Committee has examined this issue and has proposed revised norms for various levels in the Central Government. These revised norms are placed at Annexure V.
While examining the various issues relating the cadre restructuring of CSSS, it was also noted that the $14^{\text {th }}$ Finance Commission has hiked the states’ share of central taxes by 10 percentage points from the existing $32 \%$ to $42 \%$. This would have substantial impact on the financial resources available with the Central Government which may result in the scaling down of the Ministries/Departments dealing with the States.
II. (a) Change in nomenclature of Stenographer Gr.’ D’
The entry grade nomenclature of the Service is named as Stenographer Grade ‘D’. As the upper layers in the hierarchy are not designated in terms of Stenographers, the Committee finds no logic in continuing with the suffix ‘D’ with the Stenographer. The Committee, therefore, recommends that the entry grade of the Service be designated as Stenographer.
(b) Increasing the number at Steno Grade ‘D’ level:
The existing strength of Stenographers Grade D is 1324. The Committee has recommended posting them with Joint Secretaries \& equivalent, Under Secretaries and Desk Officers. With a view to correct the existing cadre structure of CSSS and keeping in view the actual requirement in the Central Secretariat, the number of posts at Stenographers Grade ‘D’, which is the entry grade to the service, may be increased from the existing strength of 1324 to 1908. The intake with respect to the increased posts will be filled in two phases. The Stenographers Grade D may also be posted in the sections. This will develop multi-skilling in the officials and will also prepare them for the grade of Assistants in CSS. Increasing the number at the entry grade would also correct the existing skewed cadre structure.
III. Decrease the number of posts at PA level:
Presently, the sanctioned strength of PA is 2627. As per the entitlement proposed by the Committee, Secretary/Special Secretary/Addl. Secretary \& equivalent and Under Secretaries \& equivalent are entitled for a PA. Considering the requirement of this
grade the Committee has proposed to decrease 727 posts at PA level and reduce the sanctioned strength to 1900. The Committee has also observed that in the last four years negligible number of vacancies under the LDCE quota has been filled. The number of nominations made during the years 2011, 2012, 2013 and 2014 through LDCE are 6, 6, 1 and Nil respectively. There are a large number of unfilled vacancies even under the SQ quota due to non availability of eligible officers in the feeder grade. The reason for this is that against 1324 sanctioned strength of Steno Grade D the strength of PA grade is 2627. This structure needs to be corrected. The Committee is of the view that the vacancies in the PA grade may be filled in the ratio of 75:25 i.e. $75 \%$ through SQ and $25 \%$ through LDCE mode by amending the Recruitment Rules which would improve the promotional opportunity in the Steno Grade D and also the requisite manpower would be available in this grade.
IV. No change in the number of posts at PS level.
Presently, the sanctioned strength of PS is 2090. As per the entitlement proposed by the Committee, Secretary/Spl. Secretary/Joint Secretary \& equivalent officers and Director and Dy. Secretary are entitled for a PS. Considering the requirement of this grade the Committee has proposed to retain the existing sanctioned strength of 2090. The Committee has also observed that in the last three years the number of vacancy under the LDCE quota has not been filled. The Committee is of the view that the vacancies in the PS grade may be filled in the ratio of 75:25 i.e. $75 \%$ through SQ and $25 \%$ through LDCE mode by amending the Recruitment Rules. This will reduce stagnation at the PA grade.
V. Increasing the number of posts at PPS level:
Presently the sanctioned strength of PPS is 780 . As per the entitlement proposed by the Committee Secretary/Special Secretary/ Addl. Secretary and Joint Secretary and equivalent in the Government of India are entitled for PPS. Considering the functional requirement, 75 posts are proposed to be increased in this grade and thereby increasing the sanctioned strength to 855 . This will also address the issue of stagnation in the feeder grade.
VI. Increasing the number of posts at Sr. PPS level:
Presently the sanctioned strength of Sr. PPS is 143 out of which $15 \%$ of the total posts of Sr. PPS and PPS can be operated at PSO level. As per the entitlement proposed by the Committee Secretary/Special Secretary/Addl. Secretary and equivalent in the Government of India are entitled for a Sr. PPS/PSO. Therefore, 68 posts are proposed to be increased in this grade keeping in view the functional requirement in the Central Government. This will also address the issue of stagnation in the PPS grade. With the said increase the sanctioned strength of Sr.PPS will be 211 .
The existing provision of operating of $15 \%$ of the total posts of Sr. PPS and PPS at PSO level shall continue.
VII Promotion upto the level of post of Joint Secretary :
The Committee also deliberated upon the demand of CSSS Associations for promotion upto the level of Joint Secretary. Keeping in view the structural and promotional hierarchy of the service and duties
\& responsibilities attached with the Service, the Committee finds no justification for creation of the post at the level of Joint Secretary in CSSS.
VIII Additional promotional opportunity for Steno Grade D:
Presently, the CSSS officials can avail of the opportunity of entry into CSS at SO level through SO/PS grade LDCE conducted by UPSC. There is a need to increase the promotional avenues for deserving CSSS officials. An opening in this regard may be provided at Assistant level of CSS through LDCE mode. As per the existing recruitment rules $10 \%$ of the vacancies in Assistant grade of CSS are filled through eligible officials from CSCS. This percentage is likely to increase in the light of the recommendations of CSS Cadre Review Committee. Stenographers Grade D, who are graduates, with 6 years of approved service may also be permitted to appear, along with UDCs of CSCS, at the LDCE for entry into Assistant grade of CSS.
IX. Issue of revival of direct recruitment in PA grade in CSSS:
Prior to first cadre structuring, direct recruitment was being made at two consecutive levels- the entry level of Steno grade D and also at the next level of PA. The $1^{\text {st }}$ Cadre Review Committee recommended that direct recruitment of PA should be discontinued. The Committee was of the view that Stenographers Grade ‘ C ‘ and ‘D’ are appointed through the same examination conducted by SSC. Both the grades have the same recruitment qualification but they are recruited to these two different grades on the basis of different stenographic skills. For Stenographer Gr. ‘C’ and Stenographer Gr. ‘D’ the minimum stenographic speed required is 100 w.p.m and 80 w.p.m. respectively. Accordingly, the Committee recommended that direct recruitment at the level of Steno Gr.’C’ should
be immediately stopped and mode of recruitment to Steno Gr. ‘C’ should be changed so that the posts are filled $50 \%$ through LDCE for Steno Gr. ‘D’ and $50 \%$ by promotion by seniority of Steno Gr. ‘D’. This would address the problem of stagnation at the level of Steno Gr. ‘D’.
The recommendation of the $1^{\text {st }}$ Cadre Review Committee was accepted and consequently direct recruitment at PA level was discontinued. Further, the recruitment rules for promotion to PA grade were modified in accordance with the recommendation.
In term of the extant provision, $50 \%$ of vacancies in PA grade are filled up by Stenographers Grade D with 6 years of approved service who qualify the LDCE conducted by SSC. The remaining $50 \%$ percent is filled through seniority quota from Steno Grade D with 10 years of approved service. If Direct Recruit (DR) PA is reintroduced this would adversely affect the promotion of Steno Grade D both under SQ and LDCE categories as the yearly vacancies available for them would get reduced to the extent of intake of DR PA.
Having regard to above facts, the Committee is not inclined to recommend re-introduction of direct recruitment at PA level in CSSS. It is of the view that mechanism of infusing young and fresh talent into CSSS already exists through the fast tract promotional avenues provided under LDCE mode both at PA and PS level under CSSS.
X. Job profile of the CSSS officers:
The duties \& responsibilities to be performed by the CSSS personnel (Sr. PPSs/PPSs/PSs/PAs/Stenographers are at present regulated by the OM No. 10/4/99-CS.II dated $1^{\text {st }}$ November, 1999. The Committee is of the opinion that the concerned Administrative Division of
Department of Personnel and Training may revise the existing duties \& responsibilities of the CSSS personnel (grade wise) keeping in view the functional requirement.
XI. Reserve in CSSS
Presently there is a provision of deputation reserve of $20 \%$, leave reserve of $3 \%$ and training reserve of $1 \%$ of the sanctioned strength of Principal Private Secretary and above in the CSSS Rules, 2010.
Provision of Reserves as provided in CSSS Rules has, however, never been operated so far perhaps for the reason that no methodology has been in place. As in other services, Reserve is to be operated in the entry grade. In the case of CSSS too, assessment of actual need for reserves and reporting of vacancies at the lowest level or Steno Grade ‘D’ should be done in advance. The Committee has accordingly deliberated the issue with reference to guidelines of CRD of DoPT. The OM dated 11.2.2013 of CRD, DoPT lays down the revised norms for reserves in the organized Group ‘A’ services as under:-
(i) Training Reserve – Not exceeding $1.5 \%$ of SDP
(ii) Leave Reserve – Not exceeding $1.5 \%$ of SDP
(iii) Deputation Reserve – Not exceeding $5 \%$ of SDP
(iv) Probationary Reserve equal to the period of probation (x) DR batch size.
The CRD guidelines on probationary reserve may not be applicable in the case of CSSS as the duration of training of DR Stenographers Grade D is only for a period of 6 weeks compared to 1-2 years in group A services. Therefore, reserves in CSSS should operate against long leave and deputations only. In parity with the provisions under
CSS Rules, the Committee recommends that a reserve of $8 \%$ of the revised cadre strength of PA and above levels in CSSS should be operated at Stenographers Grade D to meet the shortfall and requirement of manpower across Ministries/Departments. Other than Stenographers Grade D, reserves in CSSS should not be maintained in any other grade. CSSS Rules, 2010 should accordingly be amended.
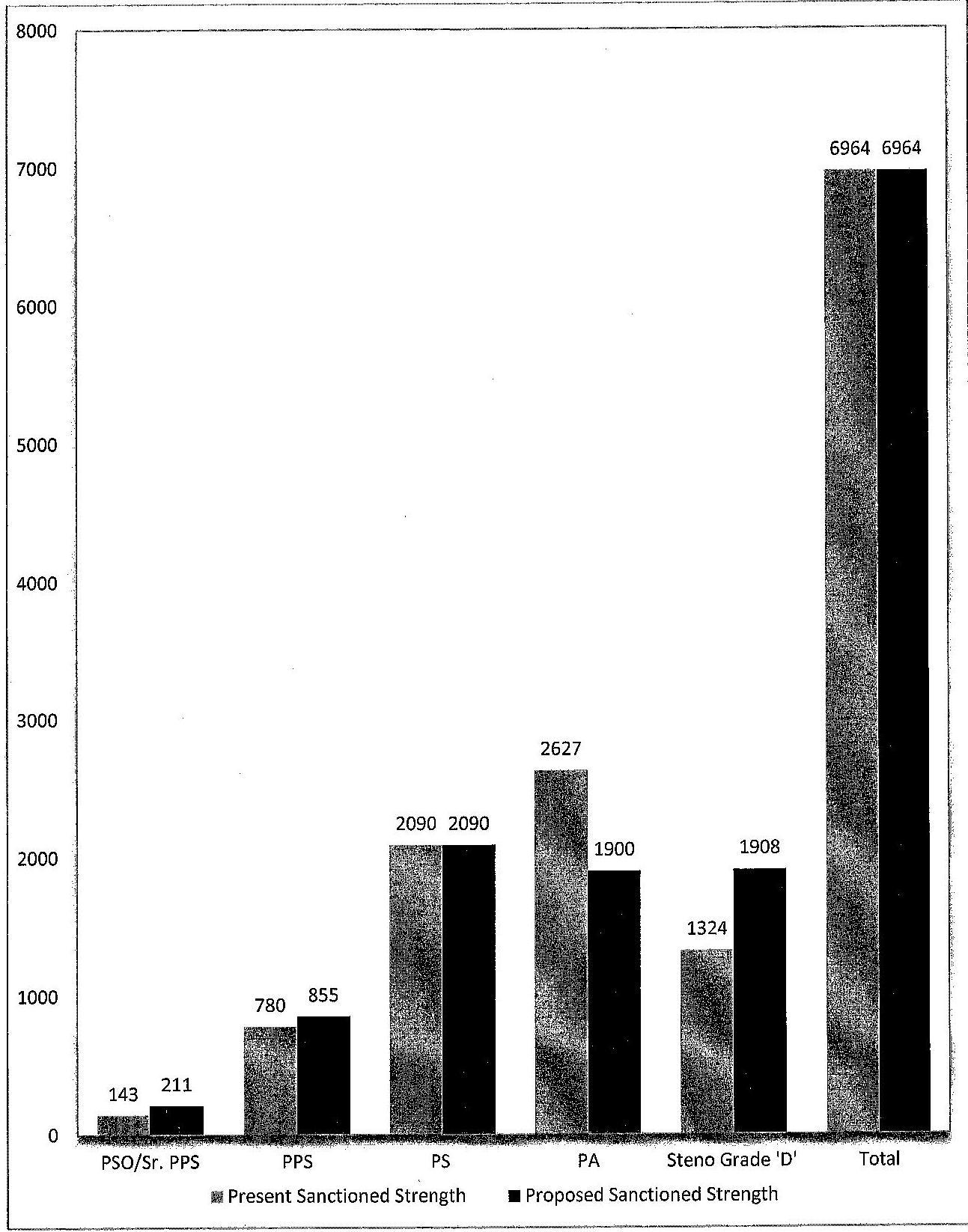
XII. Revised cadre strength
Status of CSSS Personnel : Proposed Requirement
| Sl. No. |
Grade of CSSS officers |
Present Sanctioned strength |
Proposed |
|---|---|---|---|
| 1 | PSO/Sr. PPS |
143 | 211 |
| 2 | PPS | 780 | 855 |
| 3 | PS | 2090 | 2090 |
| 4 | PA | 2627 | 1900 |
| 5 | Steno Gr. D | 1324 | 1908 |
| 6 | Total | 6964 | 6964 |
XIII. Financial Implication: There will be a savings of Rs. 1,28,77,305/per month in case the cadre strength recommended by the Committee is implemented. The details are illustrated at Annexure VI.
XIV. Residency Period
The CSSS Rules, 2010 provide for a residency period or qualifying service in a grade as an eligibility criterion for promotion to the next
grade. The residency period is specified for each grade and is computed on the basis of approved service rendered in the feeder grade. Residency requirements for promotion from one grade to the next higher grade are as under:-
| From | To | Mode of Recruitment | Residency period for promotion |
|---|---|---|---|
| Senior Principal Private Secretary (Sr.PPS) |
Principal Staff Officer (PSO) NFSG Grade | Promotion | 5 years of approved service in Sr.PPS grade Or 10 years combined regular service in PPS and Sr.PPS grade out of which 3 years in Sr.PPS grade. (The proposal for amending the RRs for providing 10 years combined approved service with three years regular service at Sr.PPS level is under process) |
| Principal Private Secretary (PPS) |
Senior Principal Private Secretary | Promotion | 5 years of approved service in PPS grade |
| Private Secretary (PS) |
Principal Private Secretary | Promotion | 6 years of approved service in PS grade |
| Personal Assistant (PA) | Private Secretary | Promotion- SQ | 5 years of approved service in PA grade |
| LDCE | 3 years of approved service in PA grade | ||
| Stenograph er Grade ‘D’ | Personal Assistant | Promotion – SQ |
10 years of approved service in Steno Grade D |
| LDCE | 6 years of approved service in Steno Grade D | ||
| Stenograph er Grade ‘D’ | Direct Recruitment |
In pursuance to the $6^{\text {th }}$ CPC, guidelines were issued by DoPT vide OM dated 24.03.2009 which prescribed minimum qualifying service in various grade pay for promotion to next higher grade pay. The Minimum qualifying service prescribed in the said OM relates to the actual regular service, whereas there is the concept of approved service in CSSS, which is the basis for deciding eligibility for promotion. Irrespective of the actual date of joining, the approved service in a particular date starts from $1^{\text {st }}$ July of the Select List Year (Promotion/Recruitment Year) in which an officer has been included. Therefore, in case of CSSS officers, regular or actual service in a grade may be less than the approved service at the time of being considered for promotion. Residency requirements, therefore, have to be seen in this background. The Committee is of the view that change in the existing residency period for promotion to the next grade under CSSS is not required.
Chart indicating the revised sanctioned strength of CS55 viz-a-viz the existing strength
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 2 moles of $$H_2O$$.
- $$m = 2 \, \text{mol} \times 48.015 \, \text{g/mol} = 24.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{T_1} = \frac{P_1}{T_2}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p + \Delta H_0$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law (Hess’s Law)
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p + \Delta H_0$$
- Variables:
- $$H$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law (Hess’s Law)
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p + \Delta H_0$$
- Variables:
- $$H$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$: Energy (K)
- $$E^\circ$$: Standard deviation of the energy (J)
- $$E$$: Standard deviation of the energy (J)
- $$E$$: Standard deviation of the energy (J)
- $$n$$: Number of moles of electrons transferred
- $$F$$: Faraday constant (96,485 C/mol)
- $$Q$$: Reaction quotient
ANNEXURE II
No. 15/1/2014-CS.II(A)
Government of India
Ministry of Personnel, Public Grievances and Pensions
Department of Personnel \& Training
Lok Nayak Bhawan, New Delhi – 110003.
Dated the $29^{\text {th }}$ December 2014
ORDER
Subject:-Constitution of a Committee for Cadre Restructuring of the Central Secretariat Stenographers’ Service (CSSS).
A Committee for the cadre restructuring of the Central Secretariat Stenographers’ Service with the following composition and terms of reference is constituted:
Composition:-
(i) Establishment Officer \& Special Secretary, : Chairman Department of Personnel \& Training
(ii) Joint Secretary (CS), Department of Personnel \& : Member Training
(iii) Joint Secretary (Pers.), Department of Expenditure : Member
(iv) Director (CS-II), Department of Personnel \& Training : Member Secretary
Terms of Reference:-
(i) To review the structure of CSSS cadre so as to harmonise the functional requirements with the career expectations of its members.
(ii) To assess the magnitude of stagnation in various grades of CSSS and suggest remedial measures – both short term and long term – to reduce promotional blocks and at the same time prevent gaps from building up.
(iii) To suggest measures to enhance the effectiveness of service and capacity building of its members.
(iv) To take into view the suggestions of the stakeholders, viz. participating Ministries, Associations and members of the service for cadre review.
(v) To review the entitlement of stenographic assistance to various category of officers of Government of India.
(vi) To examine any issue as referred to it by the cadre controlling authority of CSSS.
- The secretarial assistance to the Committee would be provided by the CS-II Division.
- This has the approval of Hon’ble Minister of State for Personnel.
(Ėameshwar Mishra)
Under Secretary to the Govt. of India
Tel: 24623157
Copy to:- -
Establishment Officer \& Special Secretary, Department of Personnel \& Training, Room No. 115-B, North Block, New Delhi.
- Joint Secretary (CS), Room No. 278-A, Department of Personnel \& Training, North Block, New Delhi.
- Joint Secretary (Pers.), Room No. 39-A, Department of Expenditure, North Block, New Delhi.
- Director (CS-II), Room No. 348, Lok Nayak Bhawan, Khan Market, New Delhi.
- Director (CS-I), Room No. 209, Lok Nayak Bhawan, Khan Market, New Delhi for information

3rd Floor, Lok Nayak Bhavan, New Delhi-110003, Dated the 06.02.2015
ORDER
Sub: Cadre Restructuring of Central Secretariat Stenographers’ Service (CSSS), 2015 – reg.
In partial modification of this Department’s order of even number dated 29.12.2014 on the subject mentioned above, vide which a Committee was constituted for the Cadre Restructuring of Central Secretariat Stenographers’ Service, the competent authority has approved that the said Committee would now function under the Chairmanship of the Additional Secretary (S \&V), DoPT.
The composition of the Members, the Terms of Reference of the Committee and other terms and conditions would remain as per the earlier Order dated 29.12.2014.

i. Additional Secretary (S \& V), DoPT, North Block, New Delhi (along with copy of the earlier OM dated 29.12.2014).
ii. Joint Secretary (Pers.), Department of Expenditure, North Block, Room No. 39-A, New Delhi.
iii. Joint Secretary (AT\&A), DoPT, North Block, New Delhi.
iv. Director (CS-II) DOPT, Lok Nayak Bhawan, Khan Market, New Delhi.
Director (CS-I) DOPT, Lok Nayak Bhawan, Khan Market, New Delhi.

.
Chart indicating the Associations who were called for interactive session and who presented their views before the Committee
- CSSS Group A Officers’ Association
Room No. 114-A, Krishi Bhawan
New Delhi 110001.
e-mail csssppsassn@gmail.com
2. CSSS Gazetted Officers’ Association
213, ‘A’ Wing, Shastri Bhawan,
New Delhi – 110001
e-mail csssgoassn@yahoo.com
3. Central Secretariat PPS/Sr. PPS/PSO Forum, Room No. 217, ‘A’ Wing, Shastri Bhawan, New Delhi- 110001
e-mail vk17@yahoo.com
4. Central Secretariat Stenographers Service Association, Room No. 216-D, Udyog Bhawan, New Delhi –
e-mail csss.assn@gmail.com
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Balance carbon atoms first.
- Then balance hydrogen atoms.
- Finally, balance oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
- Balance the equation:
- Balance oxygen atoms first.
- Then balance oxygen oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 2 \, \text{mol}$$
- Mass to Moles:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 18.015 g of 1 mole of $$H_2O$$.
- $$m = 18.015 \, \text{g/mol} = 2 \, \text{mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$ = Pressure (atm)
- $$V$$ = Volume (L)
- $$n$$ = Moles of gas
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁ = Pressure (atm)
- P₂ = Volume (L)
- P₃ = Moles of gas
- $$P_1V_1 = P_2V_2$$
- Equation: $$P_2V_2 = P_3V_3$$
Charles’s Law
- Equation: $$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
- Variables:
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
Boyle’s Law
- Equation: $$\frac{V_1}{T_1} = \frac{V_2}{T_2}$$
- Variables:
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
- $$T_1$$ = Temperature (atm)
- $$T_2$$ = Volume (L)
Thermochemistry
Enthalpy Change (ΔH)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$ = Heat transferred at constant pressure.
- $$q_p$$ = Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – Q_p$$
- Variables:
- $$\Delta H$$ = Heat transferred at constant pressure.
- $$\Delta H$$ = Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$ = Energy (K)
- $$E^\circ$$ = Heat (J)
- $$E^\circ$$ = Water (W)
- $$E$$ = Cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
- $$E$$ = Standard cell potential (V)
ANNEXURE – IV
No. 1 (Issue)
No.10/3/2004-CS.II (Pt. VII) Government of India Ministry of Personnel, Public Grievances and Pensions Department of Personnel and Training Lok Nayak Bhavan, Khan Market, New Delhi Dated the 28th October, 2005
OFFICE MEMORANDUM
Subject: – Cadre Structure of Central Secretariat Stenographers’ Service (CSSS) – Scale of personal staff of Secretaries/Special Secretaries, Additional Secretaries, Joint Secretaries etc.
The undersigned is directed to say that the Government had set up a ‘Group of Officers’ on Cadre Structure of Central Secretariat Stenographers’ Service (CSSS), which submitted its’ report in February, 2004. The recommendations of the ‘Group of Officers’ were considered by the Government and it has, inter alia, been decided to revise the entitlement of various categories of officers, to whom secretarial/stenographic assistance is provided from CSSS, as indicated below:-
| Sl. No. | Category of Officers | Category of Staff | No. |
|---|---|---|---|
| 1. | Secretary/Special Secretary/ Additional Secretary to Govt. of India and officers of equivalent rank working in the Ministries/ Departments of Govt. of India | (i) Sr. Principal Private Secretary (Sr. PPS) (Rs.12000-16500); or Principal Private Secretary (PPS) (Rs.10000-15200); and (ii) Steno. Grade ‘C’ (PA) of CSSS (Rs.5500-9000) | 01 |
| 2. | Joint Secretary to Govt. of India and Officers of equivalent rank working in the Ministries/Departments of Govt. of India | Steno. Grade ‘A’ & ‘B’ (Merged) (PS) (Rs.6500-10500) | 01 |
| 3. | Director/Deputy Secretary & officers of equivalent rank:- (i) working in the Ministries/ Departments of Govt. of India (ii) working in other participating attached offices of CSSS | Steno. Grade ‘A’ & ‘B’ (Merged) (PS) (Rs.6500-10500) Steno. Gr. ‘C’ (PA) (Rs.5500-9000) | 01 |
| 4. | Under Secretary & Officers of equivalent rank (i) working in the Ministries/ Departments of Govt. of India (ii) working in other participating/ attached offices (of CSSS) | Stenographer Grade ‘C’ (PA) of CSSS (Rs.5500-9000) Steno. Gr. ‘C’ (PA) (Rs. 5500-9000) or, Steno. Gr. ‘D'(Rs.4000-6000) | 01 |
- 32 –
| 5. | Desk Officer | Stenographer Grade ‘D’
of CSSS
(Rs.4000-6000) | 01 |
| — | — | — | — |
| 6. | Section Officer | Stenographer Grade ‘D’
of CSSS
(Rs.4000-6000) | As prescribed in MHA
O.M. No. 14/1/66-CS-II
dt. 23.4.66 (copy attached) |
- The above norms laying down the level of secretarial/stenographic assistance admissible to Officers may be operated flexibly as per the administrative exigencies by making suitable adjustments by the Ministries/Departments within the refixed strength of different grades of the Central Secretariat Stenographers’ Service (CSSS).
Encl. As above.
(G.S. BENDIR)
Under Secretary to Govt. of India
*ele. No. 24623157
To
All CSSS cadre authorities
All members of CSSS
Annexure V
Status of CSSS Personnel : Proposed Requirement
| Sl. No. |
Category of Officers | Existing entitlement | Proposed entitlement |
|---|---|---|---|
| 1 | Secretary/Spl. Secretary \& Equivalent rank |
PSO/Sr. PPS -1 | PSO/Sr. PPS -1 |
| PPS – 1 | |||
| PS – 1 | |||
| PA – 1 | PA – 1 | ||
| 2 | Addl Secy \& Equivalent rank | PSO/Sr. PPS -1 | PSO/Sr. PPS -1 |
| PPS – 1 | |||
| PA – 1 | PA – 1 | ||
| 3 | Joint Secretary \& Equivalent | PPS / PS -1 | PPS -1 |
| PS – 1 | |||
| Steno Gr. D – 1 | |||
| 4 | Director/ Dy.Secy \& Equivalent: in Ministries/ Department | PS – 1 | PS – 1 |
| 5 | In other participating/atta ched offices of CSSS | PA – 1 | PS – 1 |
| 6 | Under Secretary \& Equivalent: in Ministries/ Departments | PA – 1 | PA/Steno Gr. ‘D’ – 1 |
| 7 | Under Secretary in other participating/ attached offices of CSSS. | PA / Steno Gr, D – 1 | PA / Steno Gr D – 1 |
| 8 | Desk Officer / Section Officers | Steno Gr. D – 1 | Steno Gr. D – 1 |
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Balance carbon atoms first.
- Then balance hydrogen atoms.
- Finally, balance oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
- Balance the equation:
- Balance oxygen atoms first.
- Then balance oxygen oxygen atoms.
- Balanced equation: $$C_3H_8 + 7O_2 \rightarrow 3CO_2 + 4H_2O$$
Types of Chemical Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 18.015 g of water.
- $$m = 18.015 \, \text{g/mol} = 18.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$ = Pressure (atm)
- $$V$$ = Volume (L)
- $$n$$ = Number of moles (mol)
- $$R$$ = Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$ = Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁ = Pressure (atm)
- P₂ = Volume (L)
- P₃ = Ideal gas constant (0.0821 L·atm/mol·K)
- P₁/T = Pressure (atm)
Boyle’s Law
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{T_1}$$
- Variables:
- P₁ = Pressure (atm)
- P₂ = Volume (L)
- P₃ = Ideal gas constant (0.0821 L·atm/mol·K)
- P₁/T = Pressure (atm)
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$ = Heat transferred at constant pressure.
- $$q_p^2$$ = Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p + \Delta H_0$$
- Variables:
- $$q_p$$ = Heat transferred at constant pressure.
- $$q_p^2$$ = Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$ = Energy (K)
- $$E^\circ$$ = Heat (J)
- $$E$$ = Cell potential (V)
- $$R$$ = Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$ = Temperature (K)
- $$n$$ = Number of moles of electrons transferred
- $$F$$ = Faraday constant (96,485 C/mol)
- $$Q$$ = Reaction quotient
Annexure-VI
| STATEMENT OF FINANCIAL IMPLICATIONS OF PROPOSED REQUIREMENT | |||||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H |
| Name of the post | Pay Band (Rs) | Mean of Scale (Rs.) | Grade Pay | DA @ 107 | HRA@30% | TA + DA on TA | Total |
| Sr. PPS | 15600-39100 | 27350 | 7600 | 37397 | 10485 | 6624 | 89456 |
| 68 posts of Sr.PPS | 6082974 | ||||||
| PPS | 15600-39100 | 27350 | 6600 | 36327 | 10185 | 6624 | 87086 |
| 75 posts of PPS | 6531413 | ||||||
| Steno Grade D | 5200-20200 | 12700 | 2400 | 16157 | 4530 | 3312 | 39099 |
| 584 posts of Steno Grade D | 22833816 | ||||||
| Grand Total | 35448203 | ||||||
| PA | 9300-34800 | 22050 | 4600 | 28516 | 7995 | 3312 | 66473 |
| 727 posts of PA | 48325508 | ||||||
| Grand Total | 48325508 | ||||||
| Grand Difference | 12877305 |
- -Financial implication on account of increase in emolument owing to granting of NFSG scale has not been taken into account while working out the above mentioned statement.
.
Major recommendations of the $3^{\text {rd }}$ Cadre Restructuring Committee of CSSS
I. The norms for scale of CSSS personnel attached with the officers at various levels have been revised.
II. The sanctioned strength of Senior Principal Private Secretary (Sr.PPS) may be increased from the existing strength of 143 to 211 keeping in view the functional requirements. This will also address the issue of stagnation in the PPS grade.
III. The sanctioned strength of Principal Private Secretary (PPS) may be enhanced from the existing strength of 780 to 855 in view of the functional requirement. This will also address the issue of stagnation in the feeder grade.
IV. The existing sanctioned strength of 2090 of Private Secretary (PS) may be kept unchanged.
V. The sanctioned strength of Personal Assistant (PA) may be decreased from 2627 to 1900.
VI. It was observed that the existing cadre structure of the service is skewed inasmuch as the sanctioned strength at the Stenographer Grade ‘D’, which is the entry grade, is almost half of the strength at the Personal Assistant (PA) grade which has resulted in unfilled vacancies at PA level. Keeping this in view the Committee has recommended that the strength of Stenographers Grade ‘D’ be increased from the existing number of 1324 to 1908.
VII. The nomenclature of Stenographer Gr. ‘D’ be changed to Stenographer.
VIII. The Recruitment Rules for the PA \& PS grades may be revised to provide for filling up of $75 \%$ vacancies through Seniority Quota (SQ) and $25 \%$ of the vacancies through Limited Departmental Competitive Examination (LDCE) keeping in view the unfilled vacancies through the LDCE mode. This would also improve the promotional opportunities in Steno Grade and PA levels.
IX. With a view to increase the promotional avenues for deserving CSSS officials it has been recommended that Stenographers Grade ‘D’ with 6 years of approved service may also be permitted to appear in the LDCE for entry into Assistant grade of CSS.
X. Capacity building of the cadre has been addressed under the existing cadre plan which is mandatory for further promotion. Taking into account the official requirement the cadre needs to be trained bilingually. This training may be given during the first two years to ensure a well trained and efficient cadre. Stenographers Grade D may also be given opportunities to complete their graduation through correspondence course through any University to enable them to secure better career prospects.
XI. DoPT should revise the existing duties and responsibilities of the CSSS cadre keeping in view the functional requirement.
Duties of CSSS personnel
PSO/ Sr PPS Grade
(i) Management and supervision of personal section of Secretary/ Spl Secretary/ Addl Secretary
(ii) Knowledge of the key result areas, vision mission of the Ministry/ Department
(iii) Facilitating meetings
(iv) E-tracking of files and important papers
(v) Coordinating parliamentary work
(vi) Facilitating grievance handling
(vii) Handling phone calls
(viii) E-monitoring management system of important references
(ix) Preparation of minutes of important meetings taken by Secretary/ Spl Secretary/ Addl Secretary, in case directed.
(x) Handling co-ordination work.
(xi) Protocol duties
PPS Grade
(i) Management and supervision of personal section
(ii) Facilitating meetings
(iii) E-tracking of files and important papers
(iv) Coordinating parliamentary work
(v) Facilitating grievance handling
(vi) Taking dictation and typing
(vii) Handling phone calls
(viii) Maintaining engagements, preparing tour programmes and travel arrangements
(ix) E-monitoring management system of important references
(x) Preparation of minutes of important meetings taken by Joint Secretary
(xi) Handling co-ordination work.
(xii) Protocol duties.
PS Grade
(i) Taking dictations and typing
(ii) Handling telephone calls
(iii) Handling visitors
(iv) Maintaining engagements, preparing tour programmes and travel arrangements
(v) E-tracking of files and important papers
(vi) Handling parliamentary work
(vii) Diarising files and papers
(viii) E-monitoring management system of important references
PA/Steno Grade
(i) Taking dictations and typing
(ii) Handling telephone calls
(iii) Handling visitors
(iv) Maintaining engagements, preparing tour programmes and travel arrangements
(v) E-tracking of files and important papers
(vi) Handling parliamentary work
(vii) Diarising files and papers
(viii) E-monitoring management system of important references