India’s Central Secretariat Service (CSS) is poised for a significant overhaul designed to enhance efficiency and improve career progression. A recent committee has thoroughly reviewed the CSS structure, proposing reforms to better align functional requirements with the legitimate career aspirations of its members. Key recommendations include a strategic increase in manpower at higher-level posts, specifically for Assistants, Section Officers (SOs), Under Secretaries (USs), and Deputy Secretaries/Directors (DS/Dir). This aims to alleviate stagnation and create more promotional opportunities. The committee suggests phasing out direct recruitment for Lower Division Clerks (LDCs) and promoting existing LDCs to Assistant grade, streamlining the entry-level career path. A major shift towards a ‘Fast Track Career Progression Scheme’ is envisioned, replacing traditional competitive examinations with customized training courses and merit-based advancements. Residency requirements for promotions across various grades will be significantly reduced to accelerate career growth. Furthermore, guidelines for deputation are set to be liberalized, encouraging CSS officers to gain diverse experience in various autonomous bodies and other organizations. A comprehensive training regime, focusing on essential administrative skills like drafting and noting, will be made mandatory across all levels. While these proposed changes carry an estimated annual financial implication, long-term benefits in terms of efficiency and employee morale are expected, with a follow-up review recommended within five years to ensure the sustained effectiveness of these reforms.
SOURCE PDF LINK :
Click to view full document content

GOVERNMENT OF INDIA
MINISTRY OF PERSONNEL, PUBLIC GRIEVANCES AND PENSIONS
DEPARTMENT OF PERSONNEL & TRAINING

REPORT OF THE COMMITTEE
ON
CADRE RESTRUCTURING
OF
THE CENTRAL SECRETARIAT SERVICE
SHRI BHANU PRATAP SHARMA, AS & EO, DoPT
SHRI MANOJ JOSHI, JS (AT & A), DoPT
SMT. SUDHA KRISHNAN, JS (Pers), DoE
SHRI UTKAARSH R TIWAARI, DIRECTOR, CS.I, DoPT
DECEMBER 2013
2.1.2.2.2.2.2.3.3.4.1.4.
1. Definition
The definition of a function is the sum of its elements in a function space. It is called the product of their elements. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function. The product of the functions is denoted by $f(x)$ if and only if $f(x)$ is a function.
| Topic | Page No. |
|---|---|
| Preface | 1 |
| Abbreviations | 2-3 |
| Executive Summary | 4-29 |
| Chapter 1 | Introduction, Composition, TOR AND Methodology |
| Chapter 2 | Functional requirement |
| (i) Revival of DR SO in CSS | |
| (ii) Increase in the number of posts of Assistant | |
| (iii) Re-introduction of direct recruitment in LDC and change in mode of recruitment to Assistant grade | |
| (iv) DO System: Increase in manpower at SO and US levels | |
| (v) Increase in the number of post of DS/Dir | |
| (vi) JS (in-situ) posts | |
| (vii) Reserves in CSS | |
| (viii) Revised Cadre Strength & Reporting Structure | |
| (ix) Financial Implication | |
| Chapter 3 | Fast Track Promotion in CSS: Replacement of LDCE |
| Chapter 4 | Residency for promotion in CSS & CSCS |
| Chapter 5 | Deputation of CSS Officers |
| Chapter 6 | Encadrement of posts in Autonomous Bodies etc in CSS |
| Chapter 7 | Miscellaneous Issues |
| (i) Grant of Time Scale/personal upgradation to Under Secretaries | |
| (ii) NFSG to CSS Officers | |
| (iii) Lateral entry of CSSS into CSS | |
| (iv) In situ promotion to DS grade | |
| (v) RTP of CSS officers | |
| (vi) Training of UDCs/Assistants | |
| Annexures | 80-99 |
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.015 \, \text{g/mol} = 18.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{P_1} = \frac{P_1}{V_1}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – Q_p$$
- Variables:
- $$Q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law (Hess’s Law)
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – Q_p$$
- Variables:
- $$H_{\text{reaction}}$$: Heat transferred at constant pressure.
- $$Q_p$$: Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$: Energy (K)
- $$E^\circ$$: Standard deviation of the energy (J)
- $$E$$: Standard deviation of the energy (J)
- $$E$$: Standard deviation of the energy (J)
- $$n$$: Number of moles of electrons transferred
- $$F$$: Faraday constant (96,485 C/mol)
- $$Q$$: Reaction quotient
List of Tables
| Table No. | Title | Page No. |
|---|---|---|
| 1 | Changes in cadre structure after 1st and 2nd Cadre restructuring | 32 |
| 2 | Manpower position in the Secretariat prior to and after the cadre reviews | 35 |
| 3 | Existing Strength at various levels in Central Secretariat | 37 |
| 4 | Residency requirement for promotion in CSS | 42 |
| 5 | Composition of DR SOs in various grades | 42 |
| 6 | Strength of LDCs pre-2003 and now | 44 |
| 7 | Promotion from US to DS grade – present scenario | 49 |
| 8 | Proposed revised Cadre strength | 53 |
| 9 | Customized courses for fast track promotion | 59 |
| 10 | Revised promotion ratio | 60 |
| 11 | Residency Service for Promotion | 62 |
| 12 | CSS officers on deputation | 67 |
| 13 | Posts encadred inCSS since 2009 | 72 |
.
PREFACE
A Committee for the Cadre Restructuring (3rd) of the Central Secretariat Service (CSS) was constituted on 25.4.2013 with a specific mandate to review the structure of the CSS so as to harmonizing the functional needs with the legitimate career expectations of its members and to suggest measures to enhance the effectiveness of the Service.
2. The Committee has approached the issue in a holistic manner and arrived at its recommendations keeping the larger public interest in mind. We hope that the suggested measures would enhance the effectiveness of the Service and usher in better cadre management of the Central Secretariat Service in the future.
New Delhi, the lowDecember, 2013
.
Abbreviations
| CABs | Central Autonomous Bodies |
|---|---|
| CRC | Cadre Restructuring Committee |
| CAT | Central Administrative Tribunal |
| CS Division | Central Services Division (of DOPT) |
| CSCS | Central Secretariat Clerical Service |
| CSS | Central Secretariat Service |
| CSSS | Central Secretariat Stenographers Service |
| C.St. S | Central Staffing Scheme |
| DR | Direct Recruitment |
| DR SO | Direct Recruit Section Officer |
| DS | Deputy Secretary |
| Dir. | Director |
| E.O. Division | Establishment Officer’s Division |
| GP | Grade Pay |
| Gr. | Group / Grade |
| HAG | Higher Administrative Grade |
| LDCE | Limited Departmental Competitive Examination |
| LDC | Lower Division Clerk |
| MCQ | Multiple Choice Question |
| NFS | Non-Functional Scale |
| NFSG | Non-Functional Selection Grade |
| NFU | Non-Functional Upgradation |
| PA | Personal Assistant |
| PPS | Principal Private Secretary |
| PS | Private Secretary |
| RRs | Recruitment Rules |
| SAG | Senior Administrative Grade |
| SL | Select List |
|---|---|
| SO | Section Officer |
| SQ | Seniority Quota |
| SSC | Staff Selection Commission |
| Steno | Stenographer |
| Sr. PPS | Senior Principal Private Secretary |
| TOR | Terms of Reference |
| UDC | Upper Division Clerk |
| UPSC | Union Public Service Commission |
| US | Under Secretary |
Executive Summary
Third Cadre Review of the Central Secretariat Service
Introduction
Central Secretariat Service (CSS) is one of the earliest organized services in the country. The service conditions of members of the service were earlier regulated through CSS Rules, 1962 and Regulations made thereunder. The earlier rules were repealed and presently the service is regulated through CSS Rules, 2009 and Regulations made thereunder. While important structural changes have been made in the Central Secretariat several times, but CSS since its inception has remained the backbone of Central Secretariat especially at junior to middle management levels. It has been repository of institutional memory in central government working and has been playing a vital role in ensuring continuity of administration in the Central Secretariat.
- The cadre restructuring of the CSS was undertaken for the first time in October, 2003. The Second Cadre Restructuring Committee was constituted in the year 2008. After consideration of the recommendations of the Second Cadre Restructuring Committee, the Govt. issued orders in July, 2010 to implement the accepted recommendations. Subsequently, various issues relating to the service matters of CSS cropped up and it was decided by MoS (PP) that the third cadre review of CSS be undertaken forthwith to address all the relevant issues. Government, vide Department of Personnel & Training Order No. 19/2/2013-CS.I(P) dated 25.4.2013 constituted the Cadre Restructuring Committee, hereinafter referred to as the ‘Committee’. The Composition of the Committee and the Terms of Reference (ToR) are annexed (Annexure I). Briefly, the ToR read as under:
(i) To review the structure of CSS cadre along with the feeder cadre so as to harmonise the functional needs with the legitimate career expectation of its members;
(ii) To assess the magnitude of stagnation in various grades of CSS and suggest remedial measures both short term and long term so as to reduce promotion blocks and at the same time prevent gaps from building up;
(iii) To suggest measures to enhance the effectiveness of the service and capacity building of its members;
(iv) To take into view the suggestions of stake holders;
(v) To examine any issue as referred to it by the cadre controlling authority.
Page 4
Chemistry 101: Introduction to Chemical Bonding
1. Introduction
Chemical bonding is the process by which atoms combine to form molecules. This is mathematically represented by a bonds (bonds) or coils (atoms).
1.1 Introduction
Chemical bonding is the process by which atoms combine to form molecules. This is expressed as bonds (bonds) or coils (atoms).
1.2 Types of Chemical Bonds
1.2.1 Covalent Bond
Covalent bonds are formed between atoms in a chemical bond and a non-atomic ion. They are called covalent bonds.
1.2.2 Dovalent Bond
Dovalent bonds are formed between non-atomic ions in a chemical bond. They are called dovalent bonds.
1.2.3 Covalent Bond
Covalent bonds are formed between non-atomic ions in a chemical bond. They are called covalent bonds.
1.2.4 Dimmons
Dimmons are formed between non-atomic ions in a chemical bond. They are called dimmons.
1.3 Properties of Chemical Bonds
1.3.1 Bond Length
Bond length is the distance between the metal ions of the atom and the non-atomic ion. Bond lengths are the distance between the non-atomic ions of the atom and the non-atomic ion.
1.3.2 Bond Energy
Bond energy is the energy required to break a chemical bond. It is calculated by formula:
$$E = \frac{\hbar \cdot \text{cos} \cdot \text{tan} \theta}{2 \cdot \text{cos} \cdot \text{tan} \theta}$$
1.3.3 Bond Energy
Bond energy is the energy required to break a chemical bond. It is calculated by formula:
$$E = \frac{\hbar \cdot \text{cos} \cdot \text{tan} \theta}{2 \cdot \text{cos} \cdot \text{tan} \theta}$$
1.4 Applications of Chemical Bonding
Chemical bonding is a fundamental aspect of modern technology, with applications such as the use of materials with a high degree of strength and strength, and the development of new materials with a high degree of strength.
1.5 Conclusion
Chemical bonding is a fundamental concept in chemistry that has led to the development of various fields. By understanding the properties and behaviors of chemical compounds, we can better understand their properties and their interactions with other substances.
References
- Brown, T. (1977). Chemical Bonding. Academic Press.
- Brown, R. (1980). Chemical Bonding. Academic Press.
- Brown, R. (1981). Chemical Bonding. Academic Press.
Earlier Cadre Reviews in CSS
- Though CSS is one of the earliest organized services, no cadre review of the service was undertaken for long and there was lack of promotional avenues in the service. Some interim measures were adopted from time to time by way of in-situ upgradation of posts held by the officers as personal to them.
1984 – 60 DO/SO were made in-situ US
1990 – 186 SO/DO were made in-situ US
1997 – 225 SO/DO were made in-situ US and 72 US were made in-situ DS
1999 – 690 SO were made in-situ US and 184 US were made in-situ DS.
1st Cadre Review of CSS-2003
- The cadre review of CSS was undertaken for the first time in the year 2003. At that time there were four grades in the service viz. Assistant, SO, US and DS. The grades of Assistant and SO were decentralized in 33 cadres. The cadre was centralized in US and DS grades and posts in these grades were filled up as part of C.St.S. but officers posted as DS/US were treated as part of CSS cadre. However, no specified sanctioned strength was earmarked to CSS in these grades. CSS Officers were also eligible for empanelment / promotion against the grades of Dir and above under C.St.S.
- The important decisions on account of First Cadre Restructuring of CSS were as under:
(i) Senior Selection Grade designated as Director was introduced in CSS, with the cadre strength of 110. Posting of CSS officers against the posts of Dir, DS under C.St.S. was stopped.
(ii) The cadre strength of CSS in respect of other grades was fixed as under:
(a) Selection Grade (Deputy Secretary) 330
(b) Grade I (Under Secretary) 1400
(c) Section Officer 3000
(d) Assistant 4904
(iii) Direct recruitment to the SO grade in CSS and also to the Lower Division grade in CSCS was stopped.
(iv) Composition of the recruitment to Assistant grade was changed to $75 \%$ by DR, $15 \%$ by promotion and $10 \%$ by LDCE. (v) It was decided to stop direct recruitment of LDC and to reduce post of LDC/UDC by abolishing $85 \%$ LDC posts falling vacant each year. The Mode of recruitment to remaining posts of LDC was changed by way of $70 \%$ promotion from MTS through seniority and $30 \%$ through LDCE.
$2^{\text {nd }}$ Cadre Restructuring
- The Second Cadre Restructuring Committee of CSS was constituted on 16.6.2008 which gave its report in November, 2008. Its accepted recommendations were implemented in the year 2010. Broadly, it resulted in the following decisions: (i) A net increase of 160 posts at DS/Dir level to be made by diversion of posts from C.St.S. (ii) Inter-se flexibility in operating the posts of DS and Dir was introduced. (iii) Up to 40 CSS officers who are empanelled to be appointed as JS were permitted to be given in-situ promotion as JS. (iv) 1467 posts of UDC were upgraded to Assistant grade.
- The following table illustrates as to how the number of sanctioned posts in CSS at all levels have increased after the first and second cadre restructuring:
Changes in cadre structure after $1^{\text {st }}$ and $2^{\text {nd }}$ cadre restructuring
| Sl.
No. | Grades | Prevalent prior to 1st CRC | Sanctioned strength after 1st CRC | Sanctioned strength after the 2nd CRC | No. of posts encadred since then | Existing
Strength |
| — | — | — | — | — | — | — |
| 1. | JS/
JS (in-situ) | Under
C.St.S. | Under
C.St.S | Combined strength of 600
[Ceiling for Dir 220 and JS in-situ 40) | 38 | 594 |
| 2. | Dir | Under
C.St.S.
(100) | 110 | | | |
| 3. | DS | $59+292$
(in-situ) | 330 | | | |
| 4. | US | $579+767$
(in-situ) | 1400 | 1462 | 76 | 1538 |
| — | — | — | — | — | — | — |
| 5. | SO | 1595
(excl US in-
situ) | 3000 | 3000 | 96 | 3096 |
| 6. | Assistant | 4904 | 4904 | 6374 | 203 | 6577 |
| 7. | UDC \& LDC
(CSCS) | 10774 | $85 \%$ of DR
LDCs to be
abolished | No change | Nil | 4000
(approx.) |
Third Cadre Review : Methodology Adopted
- The Committee sought written submissions from the CSS Associations and also offered them the opportunity for presenting their views before the Committee. The Committee also offered opportunity to present their views to the associations of Central Secretariat Clerical Service (CSCS), which is the feeder cadre of CSS and the Central Secretariat Stenographers’ Service (CSSS). A list of Associations/Units which met the Committee and made presentations before the Committee is annexed (Annexure II). 8.1. The Committee held an interactive session on 16.7.2013 with the senior officers of some large Ministries/Departments to elicit their views on important issues. During the discussion, majority of the participants put across their views in favour of revival of direct recruitment of LDC in the Central Secretariat expressing their dissatisfaction with the work performed by outsourced staff. There were concerns over large scale transfers under RTP, deterioration in drafting \& note writing skills and requirement of strengthening of manpower at the crucial levels. As regards re-introduction of DR SOs, the views were, however, mixed. 8.2 The committee also took into consideration various views, grievances of Associations, functional requirement of the government, availability of manpower and other relevant factors before arriving at its recommendations.
Approaches to Cadre Restructuring of CSS : Functional requirement vis-àvis promotional prospects
- Cadre review of CSS and CSCS is intricately linked with functional requirement of the Government. The first point before the Committee is adequacy or otherwise of the available manpower for effective discharge of Government functions. CSS and
THIRD CADRE RESTRUCTURING OF CSS
CSCS occupy a central position in any such deliberations as they provide bulk of manpower to the Central Secretariat. Almost all the staff from US and below has been drawn from these two services. Therefore, their relative changing strength over time is a key indicator of the manpower availability in the Central Secretariat.
9.1
In the past, cadre review of CSS and CSCS was undertaken twice (and implemented in 2003 and 2010 respectively). The net effect of both cadre review exercises was increased in the sanctioned strength of CSS at the level of Assistant and above accompanied by sizeable reduction in the strength of CSCS. This has been illustrated in the table below:
Manpower position in the Central Secretariat prior to and after the cadre reviews
| Strength prior to 2003 (CSS+CSCS) | = | 8296+10774 = 19070 |
|---|---|---|
| Strength as on date (CSS + CSCS) | = | 11800+4000 = 15800 |
Further, at present there are about 2000 physical vacancies in Assistant grade. Therefore, the actual working strength in the Central Secretariat is only about 14000, which is roughly 5000 less than what was available prior to 1st Cadre Review of CSS. In addition, as per the accepted recommendations of the First Cadre Restructuring Committee, 85% of the strength of LDCs and 90% of the strength of UDCs were to be gradually abolished and only 1300 posts (approx) will remain as the final strength in these two cadres (LDC+UDC) together. Thus, the manpower availability in a few years’ time will be reduced to approximately 13000 which would be roughly 6000 less than what was available prior to 2003. Unless steps are taken to appropriately augment, there would be manpower crunch, which has already started being felt across Ministries/Departments.
9.2
As indicated above, bulk of the manpower in Central Secretariat up to the level of US and below is from CSS and CSCS. CSS also provides almost half the strength at DS/DIR level, remaining half being appointed under Central Staffing Scheme (C.S.S.). The appointment at JS and above is almost exclusively through C.S.S.
9.3
The sanctioned strength of manpower, drawn from CSS or CSCS or through C.S.S., at various levels is given in the following table:
Page 8
Existing strength at various levels in Central Secretariat
| Grades | Existing Strength |
|---|---|
| Secretary | 82 |
| Addl. Secretary | 76 |
| JS | 293 |
| DS/Dir | 1200 |
| US | 1538 |
| SO | 3096 |
| Assistant | 6577 |
| UDC & LDC (CSCS) | 4000 |
It is, however, noted that the table above represents core of the Central Secretariat staff. There are officers at various levels drawn from different sources/services viz. from technical services etc. but such officers only represent a small percentage of the total staff deployment in Central Secretariat.
- The workload in the Central Secretariat has gone up considerably over the last decade on account of, inter alia, following reasons:
- (i) New Ministries/Departments have been created;
- (ii) Government has been implementing various flagship schemes resulting in the need for more manpower to monitor the schemes;
- (iii) RTI Act related work; and,
- (iv) The Plan Budget of the Ministries/Departments has gone up considerably.
10.1
While the workload of Ministries/Departments has gone up over the years, there has been an overall reduction in manpower especially at lower levels. The shortage of manpower has been felt across the Ministries and there has been demand from all quarters to increase the staff availability, especially at lower levels.
10.2
The restructuring exercise of CSS and CSCS should look at a structure of the services which is sustainable in the long term keeping in view the functional aspects, reporting hierarchy and reasonable career opportunities. CSS follows a pyramidal structure with broadly 2:1 ratio at every level. The size of the CSS cadre at present is approximately 11800 of which 6577 are Assistants. With 3% annual attrition on account
THIRD CADRE RESTRUCTURING OF CSS
of retirement, approximately 350 annual intake of DR Assistants would be required. In a pyramidal structure, unless promotions are entirely by merit based selection, there is bound to be stagnation at each level. The number of posts of DS/Dir of CSS is 600. With 350 annual intake of DR Assistants, 600 posts of DS/Dir would never be sufficient for the career aspirations of these Assistants for reaching DS level or above. In the past, till 1999, severe stagnation took place in CSS. To redress that situation, a large number of posts were created at senior levels in CSS and also the size of the CSS cadre was increased substantially. In the last 15 years, due to these measures, stagnation in the CSS has reduced considerably. However, a stage has reached where it is no longer possible to keep taking ad-hoc measures to remove stagnation but to restructure CSS so as to provide reasonable career opportunities in harmony with functional requirements of the Government.
10.3 For a sustainable cadre structure of CSS, there is a need to:
(i) reduce the annual recruitment of DR Assistants;
(ii) increase the number of posts above Assistants; and
(iii) introduce faster promotional opportunities to the meritorious.
Along with the above, there is a functional requirement in the Central Secretariat to increase total manpower, particularly at lower levels. In addition, the Central Secretariat is facing acute space constraints. A large number of posts have been created at middle and senior levels along with increase in number of Departments. However, the space available in Central Secretariat has hardly increased in the last two decades. Increasing the number of posts, particularly at middle management level would lead to further space crunch. Presently at DS/Dir level, if new posts are created, there would not be any space to accommodate them.
10.4 Keeping the above requirements in perspective, it is felt that there is a need to reduce annual intake of DR Assistants so as to provide reasonable career progression to them. The requirement of increasing manpower therefore, should be met by reintroducing direct recruitment of LDCs in a limited number. This would have dual advantages, first of augmenting manpower at lower level and also of reducing need of the DR Assistants. Only with reintroduction of DR at LDC level could CSS cadre be restructured to a sustainable cadre structure. The ratio of DR versus promotees at Assistant level also needs to be changed accordingly to reduce direct recruitment of Assistants.
10.5 There is a need to increase the number of Desk Officers who should work without any Assistant/UDC/LDC reporting to them, particularly in policy desks where the volume of work is less but need for quality inputs are more. This would increase the
Page 10
manpower at SO/US level thereby improving functionality, promotion opportunities and reduce the required number of reporting Assistants/UDCs.
10.6 Many of the Ministries/ Departments resorted to engaging outsourced staff to function as Data Entry Operators or engaging officials of autonomous bodies/ PSUs under them besides hiring the services of retired officials as Consultants. Barring Consultants, the other outsourced staff and officials from PSUs mainly function as auxiliary staff and they cannot be expected to handle files or provide value addition in disposal of the work. Therefore, there is a need to replenish the manpower at the lower levels not only to cope with the increased work in the Central Secretariat but also to provide sustainable strength to the institutional memory and level of commitment, which could not be expected from the outsourced staff. Another important consideration is noting \& drafting skills of the available manpower which need to be improved.
11. Keeping the above scenario in perspective, the Committee has examined the following possible options to increase quality manpower at the Central Secretariat and also to improve promotional opportunities available to them:
(i) Re-introduce DR SO
(ii) Increase the number of posts of Assistant from the existing 6577
(iii) Re-introduce direct recruitment of LDCs
(iv) Recognize and augment desk officer system with increase in manpower at SO/US levels
(v) Increase the number of DS/Dir
(vi) Increase in the number of JS (in-situ)
(vii) Operate reserves in CSS
(viii) Introduce customized courses for fast track career progression
Introduction of DR SOs
12.1. The Committee has examined the issue relating to reintroduction of DR SOs taking into account the demands of some Associations and also the views of Ministries/Departments – some recommended re-introduction of DR SOs while others opposed it. The Committee observed that:
(i) The $1^{\text {st }}$ Cadre Restructuring Committee had noted that direct recruitment of SOs in the CSS had given rise to endless litigation over inter se
THIRD CADRE RESTRUCTURING OF CSS
seniority between DR and Promotee SOs. The first CRC had strongly opined that the direct recruitment at two consecutive levels of SO and Assistant was an impediment in the smooth administration and management of the CSS Cadre.
(ii) It was difficult to fill up DR SO vacancies in the past due to UPSC recommending lesser number of candidates for appointment against the vacancies reported to them and high attrition rate among the recommended candidates as they continued to try for Group ‘A’ service in the subsequent Civil Services Exams.
(iii) DR SOs used to constitute only 5% at SO level when the component of DR SO was 20%.
(iv) While DR SOs constituted less than 2% of the total cadre strength of the CSS, they dominate the numbers in the grade of Dir and JS in-situ.
(v) Reintroduction of DR SO will cause delay in the promotion from Assistant grade to SO grade.
The only argument in favour of DR SO is having young quality manpower in Central Secretariat. However, the quality of working in the Central Secretariat can be improved with the introduction of fast track career progression on the basis of performance in customized courses in CSS. Having regard to all the facts and circumstances, the Committee does not recommend re-introduction of DR SO in the Central Secretariat Service.
The Committee does not recommend re-introduction of DR SO in the CSS
Increase the number of posts of Assistant from the existing 6577
12.2. To increase manpower in Central Secretariat, one of the options before the Committee is to consider increase in the number of posts of Assistant. However, any increase in the number of Assistant beyond the present strength of 6577 will result in more delayed promotions in future. The Committee therefore, recommends that the number of Assistant should not be increased. To increase the availability of manpower, the Committee has separately examined and recommended re-introduction of direct recruitment of LDCs and increasing the number of posts of Desk Officers at SO/ US levels. To reduce stagnation in the grade of Assistant, the Committee has separately recommended reduction in the DR element from the present 75% to 60%, along with re-
Page 12
THIRD CADRE RESTRUCTURING OF CSS
introducing LDC grade in a limited number. As the direct recruitment of LDCs was stopped after 2003, for some years there would not be any eligible UDCs for promotion to Assistant level. Therefore, the Committee recommends that any vacancy remaining unfilled due to non-availability of eligible UDCs for promotion from seniority quota/Exam quota should be diverted to DR quota in the next examination year without any carry forward.
The Committee does not recommend increasing the strength of Assistants
Re-introduction of LDC and change in mode of recruitment to Assistant grade
12.3.
During the pre-2003 period, the combined strength of LDC/UDC was more than 10000 in the Central Secretariat and consequent to the stoppage of Direct Recruitment in the grade of LDC, their strength has shrunk to less than 4000 of which there are 3700 UDCs (regular + adhoc) and 300 LDCs. Out of the 3700 UDCs, DoPT proposes to give adhoc promotion to 1500 UDCs to the Assistant grade immediately to fill up vacancies in the grade of Assistant. There are about 2000 physical vacancies in the grade of Assistant as on date. Approximately 500 more UDCs will get promoted by 2015, leaving only about 1700 UDCs who will also get promoted to Assistant grade over a period of time against the revised proposed promotion quota of 40%. There will be no promotion to the UDC grade immediately as the newly recruited LDCs will be eligible for promotion after 8 years of residency service.
12.3.1.
Present strength of 300 LDCs have mostly been promoted from erstwhile Group ‘D’/MTS. There is a need to increase manpower at the lower/base level not only to cope with the increased work but also to provide sustainable strength to the institutional memory and to improve level of commitment. Since the overall strength of Assistant should not be increased further, the alternative for strengthening the manpower at the lowest rung will be to re-introduce direct recruitment of LDC, though in a limited manner to cater to the manpower requirement at the section level. However, it will be equally essential to provide reasonable career opportunities to direct recruit LDCs. 40% of the vacancies in the Assistant grade should be filled up from UDC grade, which works out to approx. 200 per annum. Therefore, there is a need for recruiting at least this number in LDC grade. By keeping provision for higher attrition in the grade, it should be increased to about 250 LDCs. The intake of 200-250 LDCs annually, is suggested to arrive at the sustainable number of about 2000 LDCs and equal number of
Page 13
THIRD CADRE RESTRUCTURING OF CSS
UDCs in future. Allocation of these LDCs should be made mostly to regulatory Ministries/Department where the correspondences and diarizing work etc. are high.
12.3.2.
Presently, recruitment to Assistant grade in DR:SQ:LDCE is in the ratio of 75:15:10. In order to reduce future stagnation in the Assistant grade, the Committee recommends changing the mode of recruitment to Assistant grade in the ratio of 60:20:20 [DR: SQ: LDCE/EXAM]. Such a measure will not only improve the career prospects of DR Assistants in the long term as there would be less intake of DR Assistants but will also afford reasonable career opportunities to the direct recruit LDCs.
The Committee recommends re-introduction of direct recruitment in LDC grade in a limited manner
Strengthening of Desk Officer system: Increase in manpower at SO & US levels
12.4.
As a consequence of reduction in manpower at lower levels and creation of posts in CSS at higher levels in the earlier two Cadre Reviews and continuous encadrement of newly created posts into CSS, career prospects for CSS officers have improved significantly. However, on the other hand it has led to staff crunch at certain functional levels. The Committee notes that in the first two cadre reviews of CSS, the primary emphasis was on addressing stagnation. The increased strength of CSS officers was distributed largely on pro-rata basis among participating cadres to be utilized by them as per their requirement.
12.4.1
Examining all the connected issues to find out some alternative mechanism and to address the shortage of manpower at SO and US levels, the Committee examined the first ARC report on the Machinery of the Government of India and its Procedures of work pursuant to which the Desk Officer System came into existence since the year 1973. The idea of Desk Officer System was to reduce layers of Government. The Desk Functionary was required to examine and put up the cases to DS/Dir/JS. The system also envisaged that every Ministry/Department should take steps that all future expansion in the administrative set up in connection with the functions or activities should be modelled, to the extent possible, on the desk officer pattern and effort should be made to avoid the expansion of the conventional set up in respect of new functions or activities.
Page 14
12.4.2. As of now, there are 1538 USs and 3096 SOs (total 4634) in the CSS. A number of SOs and USs are working on desk pattern i.e. without the support of conventional Section staff and reporting directly to DS/Dir. There is a need for recognizing and formalizing the structure of Desk Officer System in the Central Secretariat. Desk Officer System supported by IT enabled environment, should be extended to all Ministries/ Departments. The Committee recommends that 15% of SO/US level officers should work on the Desk Officer pattern. The combined strength of SO & US is presently 4634 and 15% of these i.e. 695 posts should be created as desks which will be of the levels of SO and US. The figure of 695 may be further split into the ratio of 1:2 for US and SO respectively and thus additional posts of 232 as US Desk and another 463 posts at the level of SO, known as Desk Officer may be created in the Ministries/Departments in CSS. The Committee recommends that an increase of 695 posts at US and SO level is the maximum additional number of posts that Central Secretariat Service could have in the present scenario. Considering the total strength of 6577 at Assistant level and the overall structure in Central Secretariat, these numbers should suffice for the next 10 years.
The Committee recommends an increase of 463 posts at SO and 232 posts at US level to be operated on desk officer pattern which should suffice for the next 10 years.
Increasing the number of posts of DS/Dir
12.5. There has been a strong demand from Associations to increase DS/Dir posts in CSS to 800-1000 to reduce delay in promotion from US to DS grade. At present almost 50% of the DS/Dir are from CSS. Most of the Ministries have been opposing the idea of tilting the percentage of DS/Dir in favor of CSS. The Committee deliberated over the issue of 1:1 ratio between CSS and C.St.S. and felt that to have officers of varied experience and skills in Central Secretariat and to provide an equitable opportunity to Group ‘A’ Central Services and AIS, the ratio of 1:1 between C.St.S. and CSS at DS/Dir level should be maintained.
12.5.1 While there does not exist functional justification for increasing the existing strength of DS/Dir, the issue is important for career expectation of CSS officers. It is observed that there is delay in promotion from US to DS grade. The time taken for promotion from US to DS grade is 10-11 years.
Page 15
12.5.2. It is felt that there is a need to have additional manpower at DS/Dir levels for reducing delay in promotion from US to DS grade. Having regard to all the relevant facts, the Committee recommends that a total of 150 posts in the grade of DS/Dir be created in the Central Secretariat and this should be divided in the ratio of 1:1 between C.St.S. and CSS to maintain the existing balance between the two streams. Accordingly, a total of 675 posts of DS/Dir each for CSS and C.St.S. would be available in the Central Secretariat. However, out of 675 posts available for CSS, the ceiling in force i.e. 220 for Dir and 40 for JS (in-situ) should continue to be maintained. In addition, as the number of posts for sections is not being proposed to be increased, the increased number of posts of DS/Dir would need to be operated in desk pattern, with desk officers of US/SO level reporting to them for policy work rather than traditional section. The Committee recommends that 1350 is the maximum number of DS/Dir that should be there in the Central Secretariat for the next 10 years.
12.5.3 The increase in work load in different Departments is not uniform. The distribution of additional posts at DS/Dir, US and SO level to different Departments should be based on the increase in work load in each Department. Departments may send proposals for increase in posts at DS/Dir, US and SO levels to Department of Expenditure based on the increase in work load. A Committee consisting of JS (Pers), Department of Expenditure and JS (AT\&A), DoPT should examine the proposals and recommend them to the Department of Expenditure. Department of Expenditure may follow the usual procedure of creation of these posts within the overall ceiling of 1350 posts at DS/Dir level, 1770 posts at US level and 3559 posts at SO level. Initially, proposals should be invited from all the departments and the first round of this exercise should be carried out in a time bound manner. Once a post is created in a Department by the Department of Expenditure, DoPT should promote and post officers to man these posts. The present mechanism for distribution of post at DS/Dir level between Central Secretariat Service and Central Staffing Scheme by Establishment Officer in DoPT may continue. Posts at US and SO level in Departments for general secretariat services should only be created to be encadred for CSS officers. The proposals for creation of posts other than the above may follow the present system of approval of Department of Expenditure on case to case basis.
12.5.4. The Committee recommends that as the number of posts for sections is not being proposed to be increased, the increased number of posts of DS/Dir, US and SO would need to be operated in desk pattern, with desk officers of US/SO level reporting to DS/Dir for policy work rather than traditional section. The Committee recommends that 1350 DS/ Dir, 232 US and 463 SO would suffice for the needs of next 10 years and is the maximum number that should be there in the Central Secretariat.
THIRD CADRE RESTRUCTURING OF CSS
12.5.5. The Committee also recommends that there is an urgent need to augment the space available in Central Secretariat. The present availability of space is not adequate and with an increase of posts at DS/ Dir, US & SO levels, additional building space would be essential. Ministry of Urban Development should take necessary action for construction of new buildings for Central Secretariat. NBCC is constructing some new office complexes at East Kidwai Nagar in New Delhi. Government should purchase necessary office space from NBCC in that complex.
The Committee recommends that a total of 150 posts in the grade of DS/Dir may be created in the Central Secretariat and this should be divided in the ratio of 1:1 between C.St.S. and CSS.
JS (in-situ) posts
12.6. CSS officers empaneled for JS under C.St.S. are made in-situ JS (subject to a ceiling of 40) in SAG Grade at their current place of posting till they are placed under C.St.S.. CSS only operates in Central Secretariat, however, the posts of JS in Central Secretariat are entirely filled up under the C.St.S. Group ‘A’ service officers get the benefit of NFU in the Grade Pay of Rs.10000/- in their cadres and there is no reporting problem. However, by virtue of CSS cadre being part of the Central Secretariat, the designation of JS (in-situ) in the service creates problems of reporting. While in larger Ministries, officers appointed as JS (in-situ) are accommodated to some extent, difficulties are faced particularly in small Ministries in reporting as officers appointed as JS (in-situ) want to report to AS/Secretary as against their functional position of Dir. This leads to confusion and distortion in the working environment in the Ministries where they are posted.
12.6.1. It is therefore, felt that, as was recommended by the 2nd CRC, the right course would have been grant of NFU with a grade pay of Rs.10,000/- in CSS and this would have benefitted more CSS officers. However, since the JS (in-situ) arrangement has already been approved and in place for some time, the same could continue.
12.6.2. The posts of JS in Central Secretariat are entirely filled by C.St.S. and are not encadred for any service. The issue of encadrement of a few posts of JS in Central Secretariat for CSS was examined in the Second Cadre review also. It was decided that the posts of JS in Central Secretariat should only be filled under C.St.S. and should not be encadred for CSS or for any other service. The Committee recommends that the same should be continued. The Committee, however, felt that
Page 17
THIRD CADRE RESTRUCTURING OF CSS
there is a case for encadrement of posts dealing with general administration for CSS in other organizations including attached/subordinate offices. In this regard, a communication was sent to all the Departments to suggest posts which could be encadred at JS level in CSS in such organizations. However, no such proposal was received from any Department. The Committee recommends that posts at JS level outside Central Secretariat in other organizations including attached/subordinate offices could be encadred in CSS based on the proposals made by those Departments. These numbers would be in addition to the limit of 40 for JS in situ. A committee consisting of Secretary (Personnel), Establishment Officer and JS (AT&A) should be authorized to examine and decide on such proposals. The Committee, however, does not recommend any increase in the strength of JS in-situ from 40.
The Committee recommends that JS (in-situ) arrangement may continue with the existing ceiling of 40.
Reserves in CSS
12.7. CSS Rules, 2009 provide for 20% deputation reserve, 3% Leave Reserve and 1% Training Reserve of the sanctioned strength of US and above level posts. These reserves are over and above the sanctioned strength.
12.7.1. In terms of extant instructions, Reserve in any service should be operated at the entry grade and not at all the levels. The CRD, DoPT guidelines on maintaining reserve for Group A services provide for (i) Training Reserve of 1.5%; (ii) Leave Reserve of 1.5%; (iii) Deputation Reserve of 5% of Senior Duty Posts; and, (iv) Probationary Reserve equal to period of probation multiplied by DR batch size. It is felt that probationary reserve will not be applicable in the case of CSS as the duration of training of probationer Assistant is only 12 weeks as compared to 1-2 years in Group ‘A’ services. Therefore, the Committee recommends that against officers going on deputation, training and long leave etc., a total of 8% of the revised cadre strength of SO and above levels in CSS should be kept as reserves to be operated at Assistant grade to meet the shortfall and requirement of manpower across Ministries/Departments on the same pattern as in Central Group ‘A’ Services. The Committee also recommends that Ministries/Departments where the reserve may sometimes need to be attached would be authorized to draw salary. Other than the Assistant grade, reserves in CSS should not be maintained in any other grade. CSS Rules, 2009 should be amended accordingly.
Page 18
The Committee recommends 8\% reserves in CSS to be operated at Assistant grade.
12.8. Financial Implication: Over the next few years, there would be large reduction in the numbers of UDCs, who would be promoted as Assistant. The number of DS/Dir, US and SO working on desk pattern would increase. Direct recruitment of LDCs and their promotion as UDC to fill the posts of LDC/UDC would take almost 10 years. Therefore, on net basis, there would be no additional expenditure as a result of cadre restructuring. Details are given in Annexure III.
13. Revised Cadre Strength :The Committee recommends following revised strength in CSS/CSCS and including DS/Dir of C.St.S. as under:
Proposed Revised Cadre Strength of CSS/CSCS
| Designation | Strength | Total | |
|---|---|---|---|
| Present | Proposed | ||
| LDC + UDC | $300+3700$ | $2000+2000$ * (over the years) |
$4000^{*}$ |
| Assistant | 6577 | 6577 | 6577 |
| SO | 3096 | $3096+463$ (Desks) | 3559 |
| US | 1538 | $1538+232$ (Desks) | 1770 |
| DS/Dir | 600 | 675 | 675 |
Note: * There are 3700 UDCs (regular + adhoc) and 300 LDCs. Out of the 3700 UDCs, DoPT proposes to give adhoc promotion to 1500 UDCs to Assistant grade immediately to fill up vacancies in the grade of Assistant. There are about 2000 physical vacancies in the grade of Assistant as on date. Approximately 500 more UDCs will get promoted by 2015, leaving only about 1700 UDCs who will also get promoted to Assistant grade over a period of time against the revised proposed promotion quota of $40 \%$. There will be no promotion to the UDC grade immediately as the newly recruited LDCs will be eligible for promotion after 8 years of residency service.
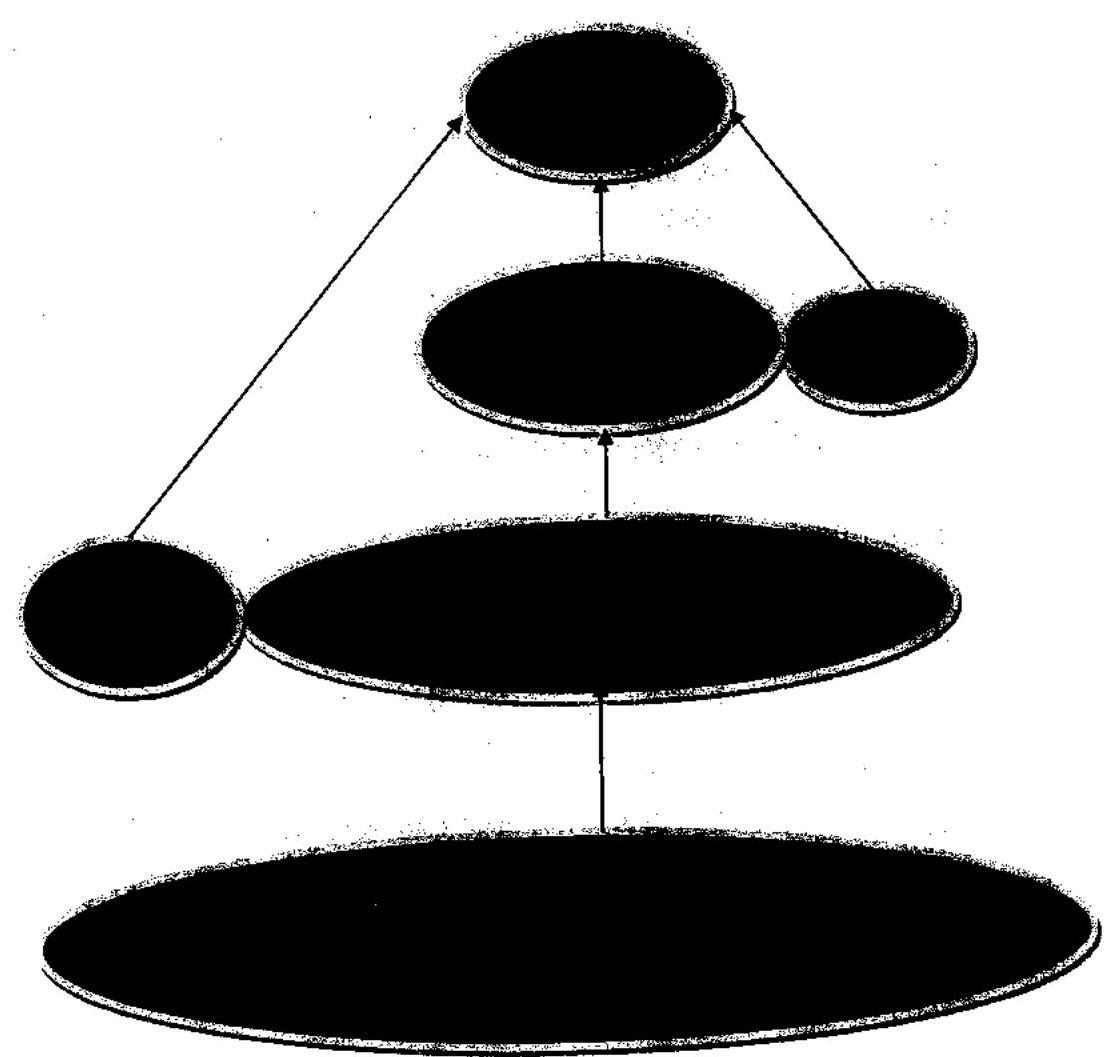
13.1. The revised reporting structure from the perspective of the entire Secretariat would be as under:
Fast Track Promotion in CSS – Replacement of LDCE
- UPSC and SSC have been conducting Limited Departmental Competitive Examinations (LDCE) for promotion to SO and UDC levels for quite some time. These Exams are MCQ based tests on various subjects that are relevant for CSS/CSCS officers. The main skill/aptitude required in CSS/CSCS is to analyze a matter and to summarize it in the form of a note to facilitate decision-making. The present LDCEs do not test these skills. Presently, there is no systematic teaching system/course material available for these exams. 14.1. It is felt that the promotion of the officials should be linked to some academic course that would provide for skill enhancement and value addition. In order to address the widely felt need for improving the quality in the Central Secretariat, the Committee recommends introduction of the scheme of customized courses for giving the meritorious officials a fast track career progression up to US grade in the CSS/CSCS if they could complete the prescribed level courses and achieve certain level of proficiency in the examination conducted by IGNOU. 14.2. Broadly, the Committee recommends that there should be customized courses on Public Policy as under:
| Level | For Promotion | Duration | Residency requirement for promotion | ||
|---|---|---|---|---|---|
| From | To | In years | Five years approved service in the lower grade | ||
| Level I (UG level) | LDC | UDC | 2 | ||
| Level II (UG level) | UDC | Asst. | 1 | ||
| Level III (PG level) | Asst. | SO | 2 | ||
| Level IV (PG level) | SO | US | 1 |
14.3. An Officer may enroll for course specified for the promotion grade without any condition of minimum eligibility service and also without any stipulation of number of attempts/years for completion. The Level I and Level III courses would be of 2 years’ duration with four semesters each and Level II and Level IV would be of one year duration with two semesters each. However, qualification with Honors degree i.e. achieving the prescribed higher percentage in the examination in the relevant grades will be compulsory for an official to be entitled for fast track career progression. However, mere qualification will not entitle him or her to be placed in a higher grade, as this would be subject to the availability of vacancy in each of the grades in a particular
year. The qualified officials in the order of merit will be treated under Exam Quota (EQ) and they will be considered for promotion along with the seniority quota officials in the ratio as given in the following table:
| Level | Existing ratio between
LDCE quota \& seniority
quota \% | Proposed ratio
between Exam
quota \& seniority
quota \% |
| — | — | — |
| LDC to UDC | $25: 75$ | $50: 50$ |
| UDC to Assistant | (75 DR): 10 LDCE : 15 SQ | $50: 50$ (out of 40\%
promotion quota
proposed) |
| Assistant to SO | $50: 50$ | $25: 75$ |
| SO to US | $100 \%$ by promotion | $25: 75$ |
The Committee recommends introduction of Fast Track Career Progression Scheme in CSS and CSCS based on BA \& MA level courses by IGNOU from LDC to US levels in place of present LDCE scheme
Revision of period of residency for promotion from one grade to the next higher grade in CSS
- Against a sanctioned strength of about 6577 Assistants, there are 4500 in position. This includes around 1200 DRs and remaining from SQ. There are 3096 SO posts, 1538 US posts and 600 DS/Dir in CSS at present and on an average 400-500 DR Assistants are recruited every year. Evidently, a ratio of $2: 1$ between successive levels is maintained in the past. In such a hierarchically steep structure, there is bound to be delay in promotions. Presently, stagnation is somewhat less as recruitment of DR Assistants has been low in the last 10 years and most of the present lot of Assistants has been promoted from the cadre of LDC/UDC, and also number of posts at higher levels have been increased considerably in the last 10 years. However, as direct recruitment in Assistant grade has increased in the last 2-3 years to meet existing vacancies and also due to increased DR recruitment ratio of $75 \%$, stagnation in CSS will increase in future.
15.1 The CSCS Rules and the CSS Rules 2009 provide the approved service as the residency period for promotion from one grade to the next higher grades as follows:
| From | To | Mode of Recruitment | Residency service (Years of approved service required in lower grade) |
|---|---|---|---|
| LDC | UDC | Seniority Quota | 8 |
| LDCE | 5 | ||
| UDC | Asst. | Promotion-SQ | 10 |
| LDCE | 6 | ||
| Assistant | SO | Promotion-SQ | 8 |
| LDCE | 5 | ||
| SO | US | Promotion | 8 |
| US | DS | Promotion | 5 |
| DS | Dir | Promotion | 5 |
15.2 After the $6^{\text {th }}$ CPC, Estt. Division of DOP\&T had issued guidelines vide OM dated 24.3.2009 prescribing minimum qualifying service in various grade pay for promotion. The minimum qualifying service prescribed in the OM dated 24.3.2009 relates to the actual regular service. There is a concept of approved service in the CSS with seniority being counted based on select list year against which an officer is promoted. In case of CSS officers, regular service is less than their approved service counted based on select year of induction/promotion. Residency requirement has to be seen in this backdrop.
UDC to Assistant Grade
15.3 CSS Regulations 2013 prescribe residency requirement of 10 years for promotion from UDC to Assistant. Earlier, under CSS Rules, 1962, the residency for promotion to Assistant was 5 years. There is a demand for reducing residency under CSS Regulations 2013 also to 5 years. 15.4 The $6^{\text {th }}$ CPC has fixed the Grade Pay of UDC as Rs. 2400 and that of Assistant as Rs.4600. The minimum residency requirement as stipulated in the guidelines of Estt (RR) for moving from GP of Rs. 2400 to Rs. 4600 is 15 years. The GP of Assistants has gone up by higher percentage, as compared to UDCs under $6^{\text {th }}$ CPC. This has resulted in increased residency requirement of 15 years for promotion from UDC to Assistant.
THIRD CADRE RESTRUCTURING OF CSS
15.5 The Associations have been raising this issue for the last 3-4 years when the CSS Regulations 2013 were being finalized. In recognition of the fact that residency of 15 years for promotion to Assistant is high, DoPT had, in relaxation of the residency requirement prescribed by the Establishment Division, had reduced it to 6 years through LDCE and 10 years through SQ for promotion from UDC to Assistant Grade under CSS Regulations 2013. The Committee accordingly found that the residency prescribed for promotion from UDC to Assistant grade is already on the lower side and, therefore, it does not recommend changing the extant provision.
Assistant to SO and SO to US grades
15.6 Residency period for promotion to various grades of CSS will have to be considered keeping in view the functional competency required of officers of a grade to effectively handle the job. The guidelines as contained in Estt. (RR) OM dated 24.3.2009 make it explicitly clear that for placement in the next higher grade pay, the time period shown therein is the minimum years of qualifying service and hence it hardly needs to be emphasized that the residency will vary from service to service depending upon various factors including the structure and size, vacancies arising as also the level of maturity and experience required for holding a particular post or grade.
15.7 The residency requirement for promotion from Assistant to SO is 5 years for LDCE and 8 years under SQ. The residency requirement at this level was same in the CSS Rules 1962 also and was not changed after 6th CPC. The residency requirement prescribed by the Estt. Division at this level is 2 years. This is quite low, the reason being that GP at Assistant level has been increased more than that at SO level after the 6th CPC. Due to the concept of approved service, many Assistants of CSS are eligible for appearing for LDCE with less than 5 years of actual service. In the case of promotion through seniority mode, the data available shows that the time taken for promotion to the SO grade through seniority would continue to be around 10 years. Moreover, it is desirable that the officer gains adequate experience and maturity to shoulder the responsibilities of SO which are supervisory levels in the Secretariat. Having regard to all these facts, the Committee feels that the existing residency of 8 years for promotion from Assistant to SO Grade is appropriate and there is no need to change the same.
15.8 The residency requirement for promotion from SO to US under the CSS Regulations 2013 is 8 years. The residency requirement at this level was same in the CSS Rules 1962 also and was not changed after 6th CPC. The residency requirement prescribed by the Estt. Division at this level is 6 years. Due to the concept of approved service, the regular services performed at SO level, that is the time taken for being eligible for promotion to US after getting the promotion as SO in practice, is less than
Page 24
THIRD CADRE RESTRUCTURING OF CSS
the residency prescribed of 8 years. Presently, SOs are promoted as US after 10 years of approved service and is likely to continue to be 10 years. Moreover, it is desirable that the officer gains adequate experience and maturity to shoulder the responsibilities of US. Having regard to all these facts, the Committee feels that the existing residency of 8 years for promotion from SO to US is appropriate and there is no need to change the same.
US to DS Grade
15.9 The Committee has noted that the residency prescribed for promotion from US to DS Grade of CSS is 5 years which is as per the guidelines of Estt. (RR). The committee examined the issue and does not recommend change in the residency already prescribed for promotion from US to DS grade.
DS to Dir
15.10 Demands have been made from various quarters of CSS officers for change in residency requirement for promotion to Dir Grade from 5 years of approved service to “5 years approved service failing which by promotion of DS with a combined service of 10 years at US & DS levels of which there shall be a minimum of 3 years of service at DS level”. The CSS Rules prescribe a residency of 5 years approved service in the grade of DS for promotion to the Dir Grade and this is as per guidelines of Estt (RR).
15.11 In CSS, as of now there are no eligible DSs with 5 years of approved service for being promoted to Dir Grade as the year 2008 was a no panel year for DS. The posts of DS & Dir in CSS are inter-changeable, and presently the vacancies in the Dir are being operated in the DS Grade and most of the officers who presently are DS, are retiring in the DS grade without completing 5 years of approved service for promotion to the grade of Dir.
15.12 Having analyzed all these issues, the Committee recommends that the residency requirement for promotion to the grade of Dir should be modified to “5 years approved service failing which a total combined approved service of 10 years at US & DS levels of which there shall be minimum regular service of 3 years at DS level”. However, since the entire process of cadre review might take time, as a stand-alone case, this issue should be taken up separately. Powers of relaxation are in built in the CSS Rules and DoPT should process the case for approval of the competent authority for relaxation and granting promotion to eligible DSs on the basis of combined approved service of 10 years in US and DS grades with a stipulation of minimum 3 years of
Page 25
regular service in the DS grade. The suggested measure would address the major grievance/demand made from various quarters of CSS.
The Committee does not recommend any change in the existing residency for promotion upto DS level.
The Committee, however, recommends that the residency requirement for promotion to the grade of Dir should be modified to 5 years approved service failing which a total combined approved service of 10 years at US & DS levels of which there shall be minimum regular service of 3 years at DS level.
Deputation of CSS Officers
- The number of officers on deputation in CSS is low at about $4 \%$ in US and above grades. The reasons for low level of deputation are:
(i) Bar on proceeding on deputation after attaining 56 years of age;
(ii) In terms of guidelines issued by D/o. Pension \& Pensioners Welfare, Central Govt. officers should be appointed in Central Autonomous Bodies (CABs) on deputation basis, only if the post is exempted from the rule of immediate absorption. A few such organizations are CCI, NHAI, NMCC, Prasar Bharati, Brain Research Centre, AIIMS, and RIMS etc.
16.1. The Committee felt that for providing CSS officers with opportunity for gaining varied experience and career enrichment, improving promotional opportunities and to enable the government organizations to benefit from the administrative experience of CSS officers, the opportunities for CSS officers to go on deputation should be increased. For this purpose, the Committee recommends that the said OM by D/o P \& PW cited above should not be made applicable for CSS officers. The age bar of 56 years should also be relaxed to 58 years. Pending withdrawal of the said OM of D/o Pension \& Pensioners Welfare, DoPT, the cadre controlling authority of CSS, should allow the officers to go on deputation without insisting on the condition that the post for which deputation is applied should be exempt from the rule of immediate absorption.
16.2 The Committee has also looked into The IAS (Cadre) Rules, 1954, which provide a liberalized regime for deputation. Under Rule 6(1), a cadre officer may be deputed for service under the Central Government or another State Government or under a company, association or body of individuals, whether incorporated or not, which
THIRD CADRE RESTRUCTURING OF CSS
is wholly or substantially owned or controlled by the Central Government or by another State Government. Similarly under Rule 6(2), a cadre officer may also be deputed for service under (i) a company, association or body of individuals, whether incorporated or not, which is wholly or substantially owned or controlled by a State Government, a Municipal Corporation or a Local Body, by the State Government on whose cadre he is borne; and (ii) an international organization, an autonomous body not controlled by the Government, or a private body, by the Central Government in consultation with the State Government on whose cadre he is borne:
16.3. It is noted that there are some conditions attached to the above deputation and, therefore, with similar conditions, the Committee recommends that a similar provision as available in rule 6(2) of IAS (Cadre) Rules, 1954 for deputation should be made in CSS Rules so that CSS officers could be allowed to go on deputation in NGOs and other organizations.
The Committee recommends that CSS Officers may be allowed to proceed on deputation to organisations which are deemed to be CABs in relaxation of D/o. P&PW’s guidelines. It further recommends a liberalized deputation regime to CSS Officers.
Encadrement of posts in CSS
- To address the problem of stagnation within the ranks of CSS especially in the grade of US, new options are to be found. The present policy of not encadering posts in autonomous organizations which are in the nature of statutory bodies, organizations registered as Societies, Committees and Commissions set up under the executive orders/Notifications of the Government requires to be reviewed. The Committee therefore, recommends that posts in organizations which are in the nature of Autonomous Bodies may be encadred in the CSS at DS/Dir level. Encadrement in such organizations at US and lower levels should not be done as CSS may itself not be able to meet that requirement. The Committee further recommends that Ministries/Departments be asked to identify posts of generalist nature in the autonomous/statutory organizations, Commissions/Committees under their administrative control for encadering them in CSS at DS/Dir level.
17.1 Service rendered in autonomous bodies is treated as Foreign Service as most of them are grantee institutions. It is, therefore, required to be stipulated that if a CSS Officer is appointed against an encadred post in an autonomous body or similar
Page 27
THIRD CADRE RESTRUCTURING OF CSS
organization, his service and the pay drawn on the post will be counted towards pension and other retirement benefits, in relaxation of CCS (Pension) Rules. Further, to avoid any hardship if an officer retires from an encadred post in any Central Autonomous Body, his pension will be finalized by the Ministry/Department under whose administrative control the organization concerned functions.
The Committee recommends that Ministries/Departments be asked to identify posts of generalist nature in the autonomous/statutory organizations, Commissions/Committees under their administrative control for encadreding them in CSS at DS/Dir level.
It should also be stipulated that the service and the pay drawn by Officers appointed against an encadred post in an autonomous body or similar organizations, will be counted towards pension and other retirement benefits, in relaxation of Note 7 below Rule 33 of CCS (Pension) Rules.
Rotational Transfer Policy for CSS Officers
- There are pros & cons both of rotational transfer policy for CSS officers from one Ministry to another. Continuity in one Ministry protects institutional memory. RTP enables officers to get varied experience and prevents forming of vested interests. RTP should balance pros & cons. Keeping the past experience in view, the Committee recommends broadly to increase the maximum period allowed in one Ministry. The existing maximum tenure policy for CSS should be revised as under:
- (i) The combined maximum tenure of Assistant/SO in a particular Ministry/Department should be revised to 10 years as against the existing 7 years.
- (ii) The maximum tenure of US should be raised to 7 years from the existing 5 years.
- (iii) There should be no change in the maximum tenure at DS/Dir level so as to keep parity with the officers coming under central staffing scheme.
- (iv) Exemption from RTP in respect of officials retiring within 2 years should continue in respect of all the grades of CSS. However, Assistants promoted from LDC grade be exempted from rotation if they are within 5 years of their retirement.
Page 28
THIRD CADRE RESTRUCTURING OF CSS
(v) DoPT should consider the requests of Ministries/Departments for retention of individual officers covered under RTP on a case-to-case basis if received with the approval of Secretary of the Ministry/Department. However, retention in such cases should not exceed 2 years.
18.1 These are the maximum tenure proposed however an officer could be transferred before that due to administrative exigencies in public interest and on the request of officers on case-to-case basis.
Training of promotee UDCs and Assistants
- There is no mandatory training programme like CSS in CSCS for promotion to the grade of UDC. The mandatory training programme for CSS starts from Level ‘A’ for promotion from UDC to Assistant. However, out of 3000 promotee Assistants in position, only 800 of them have been given level A training. There are about 3700 UDCs and most of them have not been given any training. It is recommended that training needs of these officers should be more effectively addressed both for domain rules & knowledge and noting & drafting skills.
19.1 The primary mandate of ISTM is to train officers belonging to the three Central Secretariat Services. However, the large number of promotee officers at UDC/Assistant levels which account for more than 6000, any effective training by ISTM is not practically possible. It is further felt that imparting a general rule based training will not help in bringing the required change. For better drafting skills, an officer not only should have good language skills but also good domain knowledge. In view of this, any effective training should impart both techniques of good writing skills as well as core knowledge of the subject an officer is assigned with. As Ministries/Departments deal with varied businesses, the Committee feels that Ministries/Departments are better placed in imparting training on the core areas allocated to them while simultaneously providing training for improving noting and drafting skills. Ministries/Departments should therefore, be asked to arrange periodic training for its staff appointed to the grades of UDC/Assistant through Seniority Quota promotion. Periodicity of training may be decided by the concerned Ministries; each of such training could be of 1-3 weeks in duration. ISTM should prepare a training module and develop a pool of trainers from the serving officers, retired officers and by inviting outside experts. The expenditure on this account should be met from the allocated budget of Ministries/Departments.
Page 29
Next Cadre Review of CSS
- The Committee recommends that the next Cadre Restructuring of CSS should only be undertaken 5 years after the date of sanction/approval of the Cabinet on the recommendations of the Committee to enable full implementation.
Chapter-1
Introduction, Composition, ToR and Methodology
Central Secretariat Service (CSS) is one of the earliest organized services in the country. The service conditions of members of the service were earlier regulated through CSS Rules, 1962 and Regulations made thereunder. Presently, the service is regulated through CSS Rules, 2009 and Regulations made thereunder. While important structural changes have been made in the Central Secretariat several times, CSS since inception its inception, has remained the backbone of Central Secretariat especially at junior to middle management level. It has been repository of institutional memory in Central Government working and has been playing a vital role in ensuring continuity of administration in the Central Secretariat.
- The cadre restructuring of the CSS was undertaken for the first time in October, 2003. The Second Cadre Restructuring Committee was constituted in the year 2008 and orders were issued in July, 2010 to implement its accepted recommendations. The Second CRC had also recommended that the next cadre review should not be held before 2013. Meanwhile, various issues relating the service matters of CSS cropped up and it was decided by MoS (PP) that the third cadre review of CSS may be undertaken forthwith to address all the relevant issues. Government, vide the Department of Personnel & Training Order No. 19/2/2013-CS.I(P) dated 25.4.2013 constituted the Cadre Restructuring Committee, hereinafter referred to as the ‘Committee’. The composition of the Committee and the Terms of Reference (ToR) are annexed (Annexure I). Briefly, the ToR read as under:
(i) To review the structure of CSS Cadre along with the feeder cadre so as to harmonize the functional needs with the legitimate career expectation of its members;
(ii) To assess the magnitude of stagnation in various grades of CSS and suggest remedial measures both short term and long term so as to reduce promotion blocks and at the same time prevent gaps from building up;
(iii) To suggest measures to enhance the effectiveness of the service and capacity building of its members;
(iv) To take into view the suggestions of stake holders;
(v) To examine any issue as referred to it by the cadre controlling authority.
Page 31
- Though CSS is one of the earliest organized services, no cadre review of the service was undertaken for long and there was lack of promotional avenues in the service. Some interim measures were adopted from time to time by way of in-situ upgradation of posts held by the officers as personal to them.
3.1. A brief chronology of these ad-hoc measures have been listed below:
(i) 198460 DO/SO were made US (in-situ)
(ii) 1990186 SO/DO were made US (in-situ)
(iii) 1997225 SO/DO were made in-situ USs and 72 US were made in-situ DS.
(iv) 1999690 SOs were made in-situ US and 184 US were made in-situ DS.
$1^{\text {st }}$ Cadre Review of CSS-2003
- The cadre review of CSS was undertaken for the first time in the year 2003. At that time there were four grades in the service viz. Assistant, SO, US and DS. The grades of Assistant and SO were decentralized in 33 cadres. The cadre was centralized in US and DS grades and posts in these grades were filled up as part of C.St.S. but officers posted as DS/US were treated as part of CSS cadre. However, no specified sanctioned strength was earmarked to CSS in these grades. CSS officers were also eligible for empanelment / appointment against the posts of Dir and above under C.St.S.
- The important decisions on account of First Cadre Restructuring of CSS were as under:
(i) Senior Selection Grade designated as Dir was introduced in CSS, with the cadre strength of 110 . Posting of CSS officers against the posts of Dir/DS under C.St.S. was stopped.
(ii) The cadre strength of CSS in respect of other grades was fixed as under:
(a) Selection Grade (Deputy Secretary) 330
(b) Grade I (Under Secretary) 1400
(c) SO 3000
(d) Assistant 4904
(iii) Direct recruitment to the SO grade in CSS and also to the Lower Division Clerk (LDC) grade in CSCS was stopped.
(iv) Composition of the recruitment to Assistant grade was changed to $75 \%$ through DR, $15 \%$ by promotion and $10 \%$ through LDCE.
(v) It was decided to gradually phase out LDC/UDC by abolishing $85 \%$ LDC posts falling vacant each year. The mode of recruitment to remaining posts of LDC was changed by way of $70 \%$ promotion from MTS through seniority and $30 \%$ through LDCE.
$2^{\text {nd }}$ Cadre Restructuring
- The Second Cadre Restructuring Committee of CSS was constituted on 16.6.2008 and gave its report in November, 2008. Its accepted recommendations were implemented in the year 2010. Broadly, it resulted in the following decisions:
(i) A net increase of 160 posts at DS/Dir level had been made by diversion of posts from C.St.S.
(ii) Inter se flexibility in operating the posts of DS and Dir was introduced.
(iii) Up to 40 CSS officers, who are empanelled under the C.St.S. to be appointed as JS, were permitted to be given in-situ promotion as JS.
(iv) 1467 posts of UDC were upgraded to the Assistant grade. - The following table illustrates as to how the number of sanctioned posts in CSS at all levels have changed after the first and second cadre restructuring:
Table 1: Changes in cadre structure after 1st and 2nd cadre restructuring
| Sl. No. |
Grades | Prevalent prior to 1st CRC |
Sanctioned strength after 1st CRC |
Sanctioned strength after the 2nd CRC |
No. of posts encadred since then |
Existing Strength |
|---|---|---|---|---|---|---|
| 1 | JS/ JS (in-situ) |
Under C.St.S. |
Under C.St.S. | Combined strength of 600 |
38 | 594 |
|---|---|---|---|---|---|---|
| 2 | Dir | Under C.St.S. (100) |
110 | (Ceiling of 220 for Dir and 40 for JS (in-situ)) |
||
| 3 | DS | $59+292$ (in- situ) |
330 |
| 4 | US | $579+767$
(in-situ) | 1400 | 1462 | 76 | 1538 |
| — | — | — | — | — | — | — |
| 5 | SO | 1595
(excl. US in-
situ) | 3000 | 3000 | 96 | 3096 |
| 6 | Assistant | 4904 | 4904 | 6374 | 203 | 6577 |
| 7. | UDC \& LDC
(CSCS) | 10774 | $85 \%$ of DR
LDCs to be
abolished | No change | Nil | 4000
(approx) |
Third Cadre Restructuring :Methodology Adopted
- The Committee sought written submissions from the concerned Associations of the CSS and also offered them the opportunity for presenting their view-points before the Committee. Keeping in view the concerns of allied services, the Committee also offered opportunities to the associations of Central Secretariat Clerical Service (CSCS), which are the feeder cadre of CSS and the Central Secretariat Stenographers’ Service (CSSS), to present their views. A list of Associations which met the Committee, made presentations before the Committee is annexed (Annexure II). 8.1. The Committee held an interactive session on 16.7.2013 with the senior officers of some large Ministries/Departments to elicit their views on important issues like revival of direct recruitment of LDC in the Central Secretariat, transfers under RTP, quality and requirement of strengthening manpower at the crucial levels and re-introduction of DR SO etc. 8.2 The Committee met on various occasions to deliberate and examine the issues referred to it. It took into consideration various views, counterviews, grievances of Associations, functional requirement of the government, availability of manpower and other relevant factors into consideration before arriving at its recommendations.
Chapter 2
Functional Requirement
Central Secretariat, at lower to middle levels, primarily consists of three Central Secretariat Services, namely the Central Secretariat Service (CSS), the feeder cadre to CSS, i.e. the Central Secretariat Clerical Service (CSCS) and the Central Secretariat Stenographers’ Service (CSSS). All the three services have their own respective recruitment rules and regulations issued thereunder, which govern the cadre management of each service.
- CSS is structured mainly to play a supportive role to the senior administrative levels in the policy-making and program-designing in the Central Government. In this sense it is very different from other central services as latter are organized on functional basis and they function mostly within their allotted area of competence. CSS therefore has a much wider role to play as they work in all Ministries/Departments of the Government and assigned varied nature of work though their core functions broadly remain areas of administration and establishment. With the sea changes in the challenges before the Government and delivery expectations of people from it, CSS is expected to go beyond its traditional ambit of work and bear responsibilities in the Central Secretariat that require knowledge, skill and certain measure of specialization.
Functional Requirement and Reporting Structure in the Central Secretariat
- Cadre review of CSS and CSCS is intricately linked with functional requirement of the Government. The first moot point before the committee was adequacy or otherwise, of the available manpower for effective discharge of government functions. CSS and CSCS occupy a central position in any such deliberations as they provide the bulk of manpower to the Central Secretariat. Almost all the staff from US and below has been drawn from these two services. Therefore, their relative changing strength over time is a key indicator of the manpower availability and its hierarchical structure in the Central Secretariat.
3.1. The net effect of two cadre reviews, that had been done in past, was increase in the sanctioned strength of CSS at the level of Assistant and above accompanied by sizeable reduction in the strength of CSCS. This has been illustrated in the following table:
Page 35
Table-2 : Manpower position in the Secretariat prior to and after the cadre reviews
| Strength prior to 2003 (CSS+CSCS) | $=$ | $8296+10774=19070$ |
|---|---|---|
| Strength as on date (CSS + CSCS) | $=$ | $11800+4000=15800$ |
3.2 Further, at present there are about 2000 physical vacancies in the grade of Assistant. Therefore, the actual working strength in the Central Secretariat is only about 14000, which is roughly 5000 less than what was available prior to $1^{\text {st }}$ Cadre Review of CSS. In addition, as per the accepted recommendations of First Cadre Restructuring Committee, $85 \%$ of the strength of LDCs and $90 \%$ of the strength of UDCs were to be gradually abolished and only 1300 posts (approx) will remain as the final strength in these two cadres (LDC+UDC) together. Thus, the manpower availability in a few years’ time will be reduced to approximately 13,000 which would be roughly 6000 less than what was available prior to 2003. Unless steps are taken to appropriately augment, there will be severe crisis of manpower crunch which has already started being felt across Ministries/Departments. As a result, overall manpower in the Central Secretariat has gone down – quite contrary to popular perception of the availability of increased manpower over the years. This has been depicted in the bar chart below:
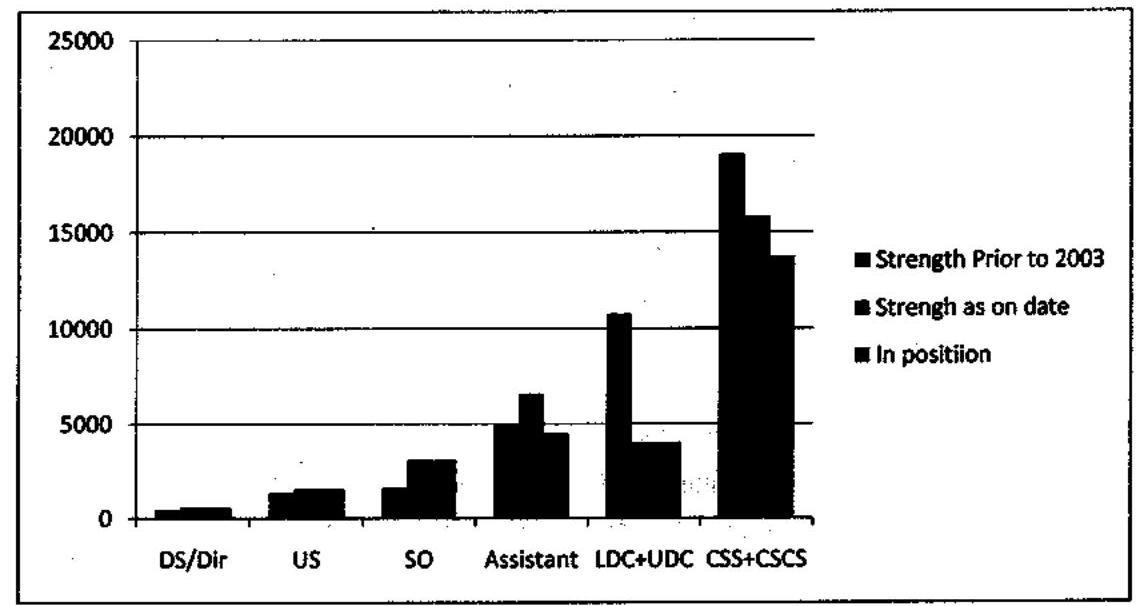
3.3 Not only there has been an overall reduction in the manpower availability in the combined cadre of CSS and CSCS, but also the composition of their relative strength has also undergone significant changes towards a more officer oriented system. This has been shown in the following charts.
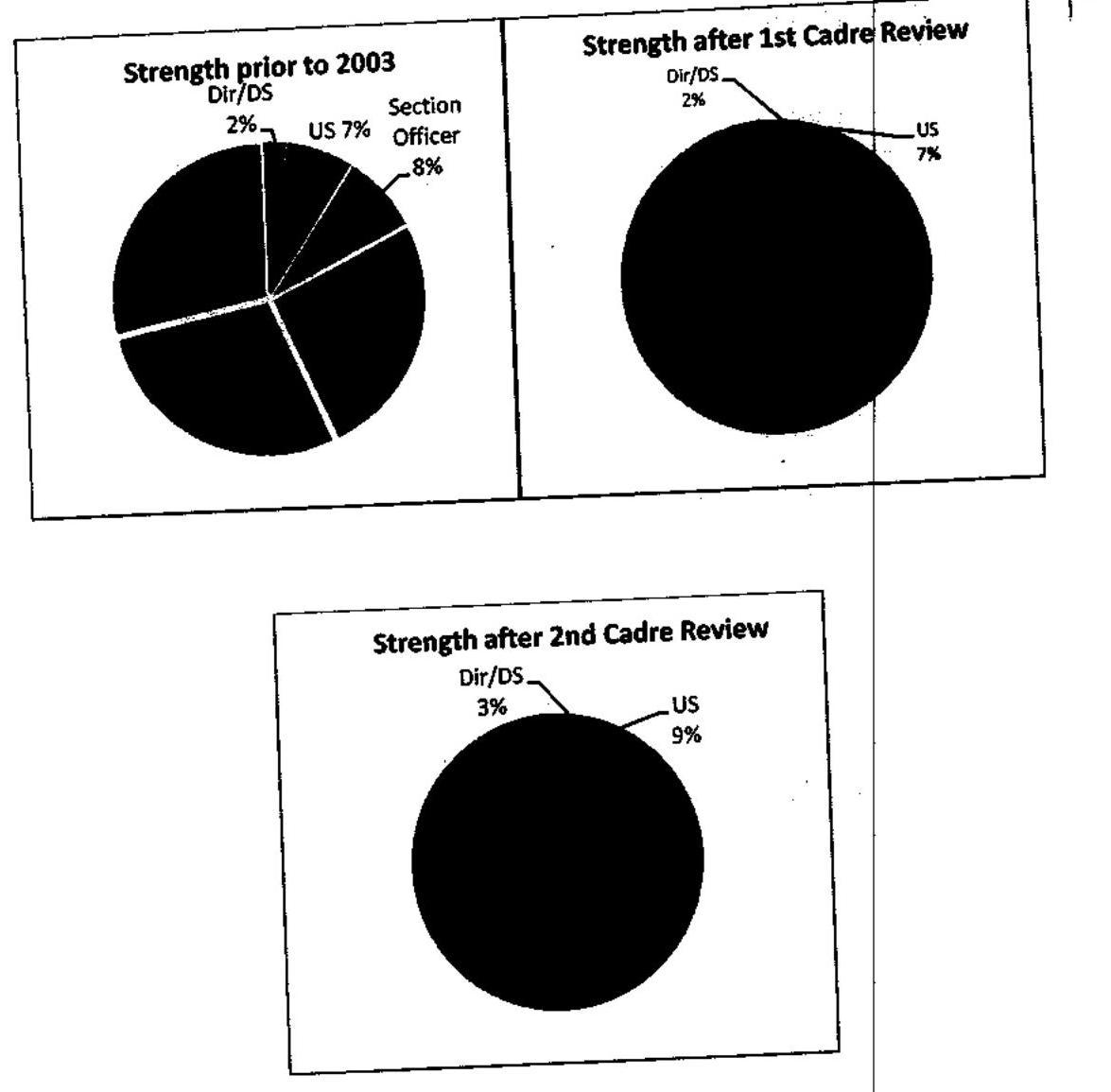
3.4 The bulk of the manpower in Central Secretariat up to the level of US and below has been drawn from CSS and CSCS. Only few of the staff deployed at these levels has been from other sources viz. Engineering Service, Economic Service or other services of technical nature. CSS also provides almost half the strength at DS/Dir level, the remaining being filled up mostly from C.St.S. The appointment at JS and above levels is almost exclusively through the C.St.S.
3.5 The sanctioned strength of manpower, drawn from CSS/CSCS or through C.St.S., at various levels is given as under:
Table-3 : Existing strength at various levels in Central Secretariat
| Grades | Existing Strength |
|---|---|
| Secretary | 82 |
| Addl. Secretary | 76 |
| JS | 293 |
| DS/Dir | 1200 |
| US | 1538 |
| SO | 3096 |
| Assistant | 6577 |
| UDC \& LDC (CSCS) | 4000 |
The table above represents core of the Central Secretariat staff. There could be officers at various levels drawn from different sources/services also viz. from technical services etc. but such officers only represent a small percentage of the total staff deployment in Central Secretariat. 3.6 Over the last decade, the work load in the Central Secretariat has gone up considerably on account of, inter alia, following reasons: (i) Creation of new Ministries/ Departments; (ii) Formulation and implementation of a multitude of Government flagship schemes resulting in the need for more manpower; (iii) Implementation of RTI Act; (iv) Litigation which has gone up considerably over recent times; and, (v) The Plan Budget of the Ministries/ Departments has also gone up by $46 \%$ in the past three years which is also indicative of increased Government activities. 3.7. The restructuring exercise of CSS and CSCS should look at a structure of the services which is sustainable in long term keeping in view the functional aspects, reporting hierarchy and reasonable career opportunities. CSS follows a pyramidal structure with broadly 2:1 ratio at every level. The size of the CSS cadre is 11800 of which 6577 are Assistants. With 3\% annual attrition on account of retirement, approximately 350 annual intake of DR Assistants would be required. In a pyramidal structure, unless promotions are entirely by merit based selection, there is bound to be
THIRD CADRE RESTRUCTURING OF CSS
stagnation at each level. DR Assistants have reasonable career aspirations of three promotions and reaching the level of DS before retirement. The number of posts of DS/Dir is 600. With 350 annual intake of DR Assistants, 600 posts of DS/Dir will never be sufficient for their career expectations. In the past, till 1999, severe stagnation took place in CSS. To redress the situation, a large number of posts were created at senior levels in CSS and also the size of the CSS cadre was increased substantially. In the last 15 years, due to these measures, stagnation in the CSS has reduced considerably. However, the stage has reached where it is no longer possible to keep taking ad-hoc measures to remove stagnation but to restructure CSS so as to provide reasonable career opportunities in harmony with functional requirements.
3.8 For a sustainable cadre structure for CSS, there is a need to:
(i) reduce the annual recruitment of DR Assistant;
(ii) increase the number of posts above Assistant; and
(iii) introduce faster promotional opportunities to the meritorious.
Along with the above, there is a functional requirement in the Central Secretariat to increase total manpower, particularly at lower levels like Assistant. In addition, the Central Secretariat is facing acute space constraints. A large number of posts have been created at middle and senior levels along with increase in number of Departments. However, the space available in Central Secretariat has hardly increased in the last few decades. Increasing the number of posts, particularly at middle management level would lead to further space crunch. Presently at DS/Dir level, if new posts are created there would not be any space to accommodate them.
3.9 Keeping the above requirements in perspective, the Committee feels that there is a need to reduce annual intake of DR Assistants so as to provide reasonable career progression to them. The requirement of increase in manpower therefore should be met by reintroducing induction of LDCs in a limited number. This would have dual advantages, first of augmenting manpower at lower level and also of reducing need of DR Assistants. Only with reintroduction of DR at LDC level could CSS cadre be restructured to a sustainable cadre structure. The ratio of DR versus Promotees at Assistant level also needs to be changed to reduce direct recruitment of Assistants.
3.10 At the same time, there is a need to increase the number of posts of Desk Officer who should work without any Assistant/UDC/LDC reporting to them, particularly in policy desks where the volume of work is less but need for quality inputs are more. This would increase the manpower at SO/US level thereby improving functionality, promotion opportunities and reduce the required number of reporting Assistants/UDCs.
Page 39
3.11 Many of the Ministries/Departments have resorted to engaging outsourced staff to function as Data Entry Operators or engaging officials of autonomous bodies/ PSUs under them besides hiring the services of retired officials as Consultants. Barring Consultants, the other outsourced staff and officials from PSUs mainly function as auxiliary staff and they could not be expected to handle files or provide value addition in disposal of the work. Therefore, there is a need to replenish the manpower at the lower/base level not only to cope with the increased work in the Central Secretariat but also to provide sustainable strength to the institutional memory and level of commitment, which could not be expected from outsourced staff. Another important consideration is drafting and noting skills in the available manpower that has of late become a major area of concern.
3.12. Keeping the above scenario in prospective, the Committee has examined the following possible routes to increase available manpower at the Central Secretariat and also to improve promotion opportunities available to them:
(i) Re-introduce DR SOs;
(ii) Increase the number of posts of Assistants from the existing 6577;
(iii) Re-introduce DR LDCs;
(iv) Recognize and augment desk officer system with increase in manpower at SO/US levels;
(v) Increase the number of DS/Dir;
(vi) Increase in the number of JS (in-situ);
(vii) Operate reserves in CSS; and
(viii) Introduce customized courses for fast track career progression.
(i) Revival of Direct Recruitment in SO Grade in CSS
4.1 Prior to first cadre restructuring direct recruitment was earlier being made at two consecutive levels- the entry level (Assistant) and also at the next level (SO – $20 \%$ of the vacancies of a year). The $1^{\text {st }}$ Cadre Review Committee recommended that direct recruitment of SO should be discontinued for the following reasons:
(i) Direct recruitment at two consecutive levels of SO and Assistant being one of the major impediments in the smooth administration and management of the CSS cadre;
(ii) Level of stagnation in the grade of Assistant;
THIRD CADRE RESTRUCTURING OF CSS
(iii) Protracted litigation in the matter of inter-se seniority between DR and Promotee SOs, which led to non-issue of regular promotion panels of US for 14 years; and
(iv) Suggestions of the Administrative Reforms Commission and the Estimates Committee (93rd Report) for abolition of Direct Recruitment of SO on account of functional inappropriateness of direct recruitment at two consecutive levels in the hierarchy of CSS.
4.3. The recommendation of first Cadre Review Committee was accepted by the Cabinet and consequently direct recruitment at SO level was discontinued. The extant CSS rules provide for direct recruitment at the level of Assistant to the extent of 75% of the vacancies arising in a year, while in the grade of SO, the posts are filled up 50% by promotion on the basis of seniority and 50% by LDCE.
Recommendations of the 2nd ARC
4.4. The Committee also took note of the fact that the 10th report of 2nd Administrative Reforms Commission, in para 7.3.3, which related to recruitment at Group B level, had recommended that each Department dealing with both the general as well as specialized services (Group B) should set up committees to examine what changes were required in the system of recruitments and promotions to these posts. Prima facie, the Commission was of the view that in order to infuse fresh thinking, a certain percentage of vacancies (say 25% every year) at the level of SO as well as for other specialized Group B posts should be filled up through direct recruitment.
4.5. The Core Group on Administrative Reforms (CGAR) viewed that all services should set up committees to examine changes required in the system of recruitments and promotions to Group B posts. It was felt that induction of SO should be encouraged through direct recruitment in Government Departments including Central Secretariat. Recommendations made by VI CPC to infuse new blood would also be taken in consideration while preparing the proposal in this regard. The Group of Ministers (GoM) has agreed with the views of the CGAR.
4.6. It may, however, be mentioned here that the VI CPC had recommended that in future, no recruitment was to be made as Assistants or Stenographers. Instead multi-skilled Executive Assistants (EAs) should be recruited who will perform the work of both the present day Assistants and Stenographers. Consequently, a proposal for introduction of Executive Assistant in the Central Secretariat is under consideration of the government. A note for the Committee of Secretaries (CoS) was also moved in
Page 41
August 2011 seeking the in-principle approval of the CoS for introduction of the scheme in the Central Secretariat.
4.7. The Committee has noted that 2nd Cadre Review Committee (July, 2010) of CSS did not consider the question of recruitment to the grade of SO. Further, in the presentations made by the Service Associations before the Committee on Cadre Restructuring of CSS on 13.6.2013, the Central Secretariat Clerical Service Association, Central Secretariat Non-Gazetted Employees Union and Central Secretariat (Promotee) Assistants’ Association did not make any demand for re-introduction of DR SO. However, Central Secretariat Group ‘A’ Officers Association, CSS Forum and DR Assistants’ Association demanded re-introduction of DR SO in the CSS. The Committee has also sought the views of Ministries/Departments. Their views are mixed – some recommended re-introduction of DR SO while others were opposed to it.
4.8. It is noted that one of the grounds given by the 1st Cadre Restructuring Committee for discontinuing the direct recruitment of SO in the CSS was that it had given rise to endless litigation over inter-se seniority between DR and Promotee SOs. On functional point, the first CRC had strongly pointed out that direct recruitment at two consecutive levels of SO and Assistant was an impediment in the smooth administration and management of the CSS Cadre. Further, the DR SOs constituted less than 2% of the total cadre strength of CSS and with less than 5% of the strength at SO level even when the component of DR SO was 20%. There was also the problem of UPSC recommending lesser number of candidates for appointment as SO as against the number of vacancies reported to them. In addition, attrition rate among DR SOs had been very high as they continued to try for Group A service in the subsequent Civil Services Exams till they crossed the eligible age limit. The Committee observes that there is no reason to expect any different situation now.
4.9. The major argument given in favor of re-introduction of DR SO is to attract young and fresh talent. The Committee has considered whether committed and talented officers can be brought about without inviting further complications in cadre management of CSS. The recurring litigation on the seniority and related issues between DR and Promotee SOs has been the bane of cadre management in CSS in the past and litigation on these and connected issues are still continuing in various judicial forum. And this has been the state of affairs despite the fact that entry of DR SO was discontinued in 2000.
4.10. As per CSS Rules, the residency for promotion from SO to US grade and above is as given in the following table:
Page 42
Table 4: Residency requirement for promotion in CSS
| From | To | Years |
|---|---|---|
| SO | US | 8 |
| US | DS | 5 |
| DS | Dir | 5 |
| Dir | JS(in-situ) | 3 |
4.11 The last DR SO joined CSS on the basis of Civil Services Exam, 2000 and presently DR SOs constitute about 2% of the total cadre strength of the CSS, but they completely dominate the numbers in the grade of Dir and JS (in-situ) as illustrated below.
Table 5: Composition of DR SOs in various grades
| Grade | CSS Officers | No. of DR SOs | % |
|---|---|---|---|
| JS(in-situ) | 34 | 34 | |
| Dir | 128 | 104 | 30% in a total strength of 600 |
| DS | 426(including ad hoc DS) | 42 | |
| US | 1538 | 23 |
Effect on the promotional prospects of Assistant on re-introduction of DR SO
4.12. In terms of extant provisions, 50% of the vacancies in the SO grade are filled up by Assistants/PAs with 5 years of approved service who qualify the LDCE conducted by UPSC and the remaining 50% is filled up through seniority quota from Assistants. If DR SO is re-introduced, there will be adverse effect on the promotion of Assistants both under SQ and LDCE categories as the yearly vacancy available for them would get reduced to the extent of intake of DR SOs. As per the tentative assessment, intake of DR SOs would delay promotion prospect from Assistant to SO grade by one year. It is also noted that promotion to SO grade is already taking 10-11 years. Assistant SL 2003 is very large and they are likely to be promoted to SO grade in SOSL 2013 and 2014. If DR SO is introduced, their inclusion in SOSL will get further delayed and they would be included well past SL 2015 only.
Page 43
4.13. Having regard to all the facts and circumstances, the Committee is not inclined to recommend re-introduction of DR SO in the Central Secretariat Service. It is of the view that the objective of infusing young and fresh talent into CSS can be achieved by introducing a system of fast track promotional avenue to deserving Assistants/SOs on completion of 5 years of service in the respective grade. The quality of manpower in the Central Secretariat can also be improved with the introduction of fast track career progression based on performance in customized courses which will ensure availability of comparatively bright and talented officers up to the level of US. The modalities of providing fast track career progression to talented officers of CSS and their seniority have been discussed separately.
(ii) Increasing the number of posts of Assistant from existing 6577
- To address to the demand of increased manpower at the lower levels, the Committee examined whether the availability of manpower at the level of Assistant could be increased beyond the present strength of 6577. The strength of Assistant prior to first cadre restructuring was 4904 and the extant CSS Rules provided for filling up of $50 \%$ vacancies through direct recruitment and the remaining $50 \%$ of vacancies through promotions from UDC grade. The strength of Assistant in CSS was not increased after the first Cadre Restructuring of CSS although mode of recruitment was changed and the intake of DR Assistant was increased from $50 \%$ to $75 \%$.
5.1 The second Cadre Restructuring resulted in increasing the strength of Assistant from 4907 to 6374 by upgrading 1467 posts of UDC. Since then many posts of Assistants in Ministries/Departments have been encadred into CSS on functional considerations and as of now the strength is 6577 .
5.2 It is informed that for promotion from Assistant to SO grade, Select Lists up to the year 2011 are being finalized shortly and as per the tentative estimates Assistants of SL 1997-2002 are likely covered in the SO SL 2012. However, Assistant SL 2003 is quite large and hence it would take more time. The average intake of Assistants is 350400 every year. Presently, stagnation in the cadre is relatively less as recruitment of DR Assistant was low in the last 10 years and most of the present strength at Assistant are from promotee cadre. However, as DR Assistant recruitment has increased in the last 2-3 years to meet vacancy and also due to increased DR ratio $75 \%$, the possibility of increased delay in promotion cannot be ruled out in future. Moreover, with 350-400 annual intake of DR Assistants, 600 posts of DS/Dir will never be sufficient for their
career expectations. Therefore, increasing the strength of Assistant beyond the existing number is not considered desirable.
(ii) Re-introduction of direct recruitment in LDC and change in mode of recruitment to Assistant grade
- Re-introduction of direct recruitment in the grade of LDC in the Central Secretariat is one of the issues referred to the Committee. The committee approached the matter as an option for strengthening the manpower at the lowest rung of sections particularly in the backdrop of increasing the strength of Assistant grade has not been considered desirable.
6.1 The Committee has noted that the First Cadre Restructuring Committee had recommended that gradually the posts of UDCs and LDCs would be abolished over the period and it was envisaged that in place of the Clerical Service, the direct recruits at the Assistant level would perform the routine tasks.
6.2. As a part of first cadre review, it was decided with the approval of the Cabinet to discontinue direct recruitment to the post of LDC and to abolish the vacant posts of LDC which are filled up through direct recruitment. Eighty Five percent of the posts of LDC were filled up through direct recruitment quota then and accordingly, after implementation of the first cadre review, 85% of the posts becoming vacant every year were being abolished. The remaining posts were filled up by promotion from the erstwhile Group D employees. Over the years, strength of LDCs in the Ministries/Departments has come down substantially and their comparative strength is as under:
| No. of LDCs prior to 2003 | Present strength |
|---|---|
| 5397 | 300 |
Table 6 : Strength of LDCs pre – 2003 and now
6.3. A few Ministries/Departments have expressed their views for reintroduction of LDCs on functional grounds. The 2nd Administrative Reforms Commission has also recommended for revival of LDC.
Page 45
191
THIRD CADRE RESTRUCTURING OF CSS
6.4. The Committee has discussed the issue of re-introduction of LDC in the Central Secretariat Clerical Service (CSCS) in consultation with Ministries/Departments. It is observed that the discontinuation of direct recruitment in the grade of LDC since the year 2003 has had a major impact on the secretariat functioning. It is further observed that—
(i) In the absence of LDCs, a number of Ministries/Departments have resorted to engaging out-sourced staff to manage some of the most basic activities of secretariat working like diary/dispatch, movement of files/papers, record-keeping, typing, file-tracking etc.
(ii) The combined strength of LDC/UDC has shrunk to less than 4000 from over 10000 in the pre-2003 period of which there are only 3700 UDCs and 300 LDCs are actually available.
(iii) There is a functional need to strengthen the institutional memory and level of commitment among lower functionaries, which cannot be expected from outsourced staff.
6.5 The Committee is of the opinion that the restoration of direct recruitment in the grade of LDC is the need of the hour. However it is also noted that with the increased use of information technology tools and progressively more officer oriented system there is a much less requirement of ministerial staff than previously and therefore the reintroduction of recruitment at the level of LDC should be limited in number and ministerial staff should mostly be utilised in regulatory ministries where the volume of correspondence/dak/diarizing work is higher viz. MHA, PMO, DoPT, Cabinet Secretariat, UPSC etc.
6.6 The Committee also observes that the requirement of LDCs/UDCs in each of the Ministries/Departments should be worked out accordingly.
Promotional prospects of newly recruited direct recruit LDC
6.7 During the pre-2003 period, the combined strength of LDC/UDC was more than 10000 in the Central Secretariat and consequent to the stoppage of Direct Recruitment in the grade of LDC, their strength had shrunk to less than 4000 of which there are 3700 UDCs (regular + adhoc) and 300 LDCs. Out of the 3700 UDCs, DoPT proposes to promote 1500 UDCs to adhoc Assistant immediately to fill up vacancies in the grade of Assistant. There are about 2000 physical vacancies in the grade of Assistant as on date. Approximately 500 more UDCs will get promoted by 2015 leaving only about 1700 UDCs who will also get promoted to Assistant grade over a period of time against the revised proposed promotion quota of 40%. There will be no promotion to the UDC
Page 46
THIRD CADRE RESTRUCTURING OF CSS
grade immediately as the newly recruited LDCs will be eligible for promotion after 8 years of residency service.
6.8. While the Committee recommends introduction of direct recruitment in LDC in a limited manner, it has also looked into the career progression opportunities made available to them. It is noted that at present there is acute stagnation in the grade of UDC and with the present ratio of 75:25 (DR/Promotee) for recruitment to the Assistant grade, UDCs of SL 1996 may at best be covered in the Assistant SL of 2013 i.e. after putting in service of about 17 years. However, if the ratio is tinkered in favor of UDCs to 60:40 (DR: Promotee), UDCs up to the year 2000 (approximately 800 UDCs) can be covered in the Assistant SL of 2013-14. In subsequent years, considering the average yearly intake of around 500 Assistants, there will be 200 vacancies through SQ/Exam quota for UDCs and adding the retirement and other vacancies, problem of stagnation in the grade of UDC can be addressed significantly. And this will in turn facilitate career progression for direct recruit LDCs as the vacancies would trickle down.
6.9 As already observed, in order to ensure adequate promotional avenues to the newly recruited LDCs, the percentage of intake of DR Assistant needs to be reduced to 60% (from the existing 75%) leaving the 40% of the vacancies in the Assistant grade to be filled up by seniority/Exam quota from the UDC grade. This will also be beneficial for DR Assistants in long run as it would reduce possible stagnation in senior grades of CSS. As such, out of 40% quota for UDCs in the Assistant grade, 20% may be earmarked for those who qualify with higher percentage the customized course conducted by IGNOU and the remaining 20% of vacancies in the Assistant grade may be filled up through seniority. To summarize, the Committee recommends changing the present recruitment ratio in the Assistant grade from existing 75:15:10 (DR:SQ:LDCE) to 60:20:20 (DR:SQ:EXAM). The existing system will, however, continue till the new arrangements are in place.
Feasible number of direct recruit LDC
6.10. The Committee felt that 40% of the vacancies in the Assistant grade should be reserved for promotion from the rank of UDC grade, which works out to approx. 200 per annum. Going by this, there would be a need for recruiting at least this number in LDC grade to provide for the feeder for the Assistant grade through seniority quota. By keeping provision for attrition, it would work out about 250 LDCs per annum. In the changed IT environment, the intake of 200-250 was suggested to arrive at the sustainable number of about 2000 LDCs and equal number of UDCs over the years. And in no case the combined strength of LDCs and UDCs in the entire Central Secretariat should exceed 4000. Allocation of these LDCs should be made mostly to
Page 47
regulatory Ministries/Departments where the correspondences and diarizing work etc. are high.
(iv) Desk Officer system: Increase in manpower at SO \& US levels
- As a consequence of overall reduction in manpower at lower levels and creation of the posts in CSS at higher levels in the two Cadre Reviews and also continuous encadrement of newly created posts into CSS on the basis of functional requirements, there has been significant improvement of career prospects for CSS officers. It has, however, led to staff crunch at certain functional levels. The Committee has noted that in the first two cadre reviews of CSS, the primary focus was on addressing stagnation. The increased strength of CSS officers were distributed largely on pro-rata basis among participating cadres to be utilized by them as per their convenience.
7.1. At present there are 1538 USs and a total of 1200 DS/Dir in Ministries/Departments of the central government out of which 600 are from CSS and another 600 under the C.SIS. Ideally, in a well-structured pyramid, it should be in the ratio of 1:2 between DS/Dir and US and the same ratio should be followed down below.
7.2. The Committee has attempted to examine all the connected issues in a bid to find out some alternative mechanism to address the shortage of manpower especially at SO and US levels. The Committee took into account first ARC’s report on the Machinery of the Government of India and its Procedures of work pursuant to which the desk officer system came into existence since the year 1973. D/o AR \& PG has made study of Desk Officer System and guidelines issued by them are being followed by Ministries/Departments. The very idea of Desk Officer System is to adopt an actionoriented system under which the Desk Functionary examines and puts up the cases directly to DS/Dir/JS. The system also envisaged that every Ministry/Department should take steps that all future expansion in the administrative set up in connection with the functions or activities should be modeled, to the extent possible, on the desk officer pattern and effort should be made to avoid the expansion of the conventional set up in respect of new functions or activities.
7.3. SOs and USs remain the pillars of Central Secretariat system in as much as they embody the tradition, experience and memory of the Secretariat besides providing continuity to administration. As of now, there are 1538 USs and 3096 SOs (total 4634) in the CSS and as per educated estimates, more than $20 \%$ of SOs and USs are working on desk pattern i.e. without the support of conventional Sections and they are reporting directly to DS/Dir. The exact data on the number of SO level officers directly reporting to DS/Dir could not be made available. The Committee feels the need for recognizing and formalizing the structure of desk officer system in the entire Central Secretariat. Desk Officer System supported by IT enabled environment, should be extended to all Ministries/Departments and they should be given adequate freedom to utilize them as per their requirements. The combined strength of SO \&US is presently 4634 and $15 \%$ of these i.e. 695 posts should be created as desks which will be of the levels of SO and US. The figure of 695 may be further split into the ratio of $1: 2$ for US and SO respectively and thus additional posts of 232 as US Desk and another 463 posts at the level of SO, known as Desk Officer may be created in the Ministries/Departments in CSS. The Committee recommends that an increase of 695 posts at US and SO level is the maximum number of additional posts that Central Secretariat Service could have in the present scenario. Considering the total strength of 6577 at Assistant level and the overall structure in Central Secretariat, these numbers should suffice for the next 10 years.
| 100 | 1000 | 10000 | 100000 | 100000 | 100000 |
|---|---|---|---|---|---|
| 100000 | 1000000 | 1000000 | 1000000 | 1000000 | 1000000 |
| 1000000 | 1000000 | 1000000 | 1000000 | 1000000 | 1000000 |
| 1000000 | 1000000 | 1000000 | 1000000 | 1000000 | 1000000 |
| 1000000 | 1000000 | 1000000 | 1000000 | 1000000 | 1000000 |
| 1000000 | 1000000 | 1000000 | 1000000 | 1000000 | 1000000 |
| 1000000 | 1000000 | 1000000 | 1000000 | 1000000 | 1000000 |
(v) Increasing the number of post of DS/Dir
- There has been a strong demand from Associations to increase DS/Dir posts in CSS to 800-1000 to reduce the delay in promotion. At present almost $50 \%$ of the DS/Dir are from CSS. Most of the Ministries have been opposing the idea of tilting this percentage in favor of CSS. Existing ratio between CSS and C.St.S.at the level of DS/Dir is $1: 1$ and it is felt that to have officers of varied experience and skills in Central Secretariat and to provide an equitable opportunity to Group ‘A’ Central Services and AIS to have an exposure to the Central Secretariat working, the ratio of 1:1 between C.St.S. and CSS should be maintained.
8.1 Presently, USs of Select Lists 2003 and 2004 are in the zone of promotion to the grade of DS and the waiting period for the USSL 2003 and onwards is around 10-11 years. The situation is likely to continue and in fact it will increase as the number of retirement in DS and above grades is decreasing in the coming years and later Select Lists of USs viz. USSL 2009 and onwards are larger. The position with reference to the existing Select Lists of US and their likely inclusion in the DS Select List has been described in the following table:
Table 7: Promotion from US to DS grade – Present Scenario
| US SL Year | Inclusion Year and size DS
SL Year wise (likely) | Years to be
taken |
| — | — | — |
| 2003 | $2013(95)^{}$ | 10 |
| 2004-2005 | $2014(103)^{}$ | 10 |
| 2006 | $2015(80)^{}$ | 09 |
| 2006-07 | $2016(76)^{}$ | 10 |
| 2007 | $2017(85)^{}$ | 10 |
| 2008 | $2018(91)^{}$ | 10 |
| 2009 (size 268) | $2019(95)^{}$ | 10 |
| | $2020(103)^{}$ | 11 |
| 2010 (size 215) | $2021-2022$ | $11-12$ |
| 2011 (size 371) | $2023-2024$ | $12-13$ |
- Numbers shown in brackets are the maximum tentative number of USs that can be included in DS SL. 8.2 It is observed that there has been an overall improvement in the promotional prospect of CSS officers over the last decade. However, there is delay in promotion from US to DS grade. 8.3 While there does not exist functional justification for increasing the existing strength of DS/Dir, the issue is important for career expectation of CSS officers. It is
THIRD CADRE RESTRUCTURING OF CSS
observed that there is delay in promotion from US to DS grade. The time taken for promotion from US to DS grade is 10-11 years.
8.5
It is felt that there need to increase posts at DS/Dir levels for reducing delay in promotion from US to DS grade. Having regard to all the relevant facts, the Committee recommends that a total of 150 posts in the grade of DS/Dir be created in the Central Secretariat and this should be divided in the ratio of 1:1 between C.St.S. and CSS to maintain the existing balance between the two streams. Accordingly, a total of 675 posts of DS/Dir each for CSS and C.St.S. would be available in the Central Secretariat. However, out of 675 posts available for CSS, the ceiling in-force i.e. 220 for Dir and 40 for JS (in-situ) should continue to be maintained.
8.6
The increase in work load in different Departments is not uniform. The distribution of additional posts at DS/Dir, US and SO level to different Departments should be based on the increase in work load in each Department. Departments may send proposals for increase in posts at DS/Dir, US and SO levels to Department of Expenditure based on the increase in work load. A Committee consisting of JS (Pers), Department of Expenditure and JS (AT&A), DoPT should examine the proposals and recommend them to the Department of Expenditure. Department of Expenditure may follow the usual procedure of creation of these posts within the overall ceiling of 1350 posts at DS/Dir level, 1770 posts at US level and 3559 posts at SO level. Initially, proposals should be invited from all the departments and the first round of this exercise should be carried out in a time bound manner. Once a post is created in a Department by the Department of Expenditure, DoPT should promote and post officers to man these posts. The present mechanism for distribution of post at DS/Dir level between Central Secretariat Service and Central Staffing Scheme by Establishment Officer in DoPT may continue. Posts at US and SO level in Departments for general secretariat services should only be created to be encadred for CSS officers. The proposals for creation of posts other than the above may follow the present system of approval of Department of Expenditure on case to case basis.
8.7
The Committee recommends that as the number of posts for sections in not being proposed to be increased, the increased number of posts of US and SO would need to be operated in desk pattern. The Committee recommends that 1350 DS/ Dir, 232 US and 463 SO would suffice for the needs of next 10 years and is the maximum number that should be there in the Central Secretariat.
8.8
The Committee also recommends that there is an urgent need to augment the space available in Central Secretariat. The present availability of space is not adequate and with an increase of posts at DS/ Dir, US & SO levels, additional building space
Page 51
would be essential. Ministry of Urban Development should take necessary action for construction of new buildings for Central Secretariat. NBCC is constructing some new office complexes at East Kidwai Nagar in New Delhi. Government should purchase necessary office space from NBCC in that complex.
(vi) JS (in-situ) posts
- CSS officers empanelled as JSs are made in-situ JS (subject to a ceiling of 40) in SAG Grade at their current place of posting till they are placed under C.St.S. CSS only operates in Central Secretariat, however, the posts of JS in Central Secretariat are entirely filled up under C.St.S. Group ‘A’ service officers get the benefit of NFU in the Grade Pay of Rs.10000/- in their cadres and hence there is no reporting problem. However, by virtue of CSS cadre being part of the Central Secretariat, the designation of JS (in-situ) in the service creates problems of reporting. While in larger Ministries, JS (in-situ) are accommodated to some extent, there are difficulties faced particularly in small Ministries in reporting as officers appointed as JS (in-situ) want to report to AS/Secretary as against their functional position of Dir. This leads to confusion and distortion in the working environment in the Ministries where they are posted.
9.1. It is therefore, felt that, as was recommended by the 2nd CRC, the right course would have been grant of NFU with a grade pay of Rs.10,000 in CSS and this would have benefitted more CSS officers. However, since the JS (in-situ) arrangement has already been approved and in place for some time, the same could continue.
9.2 The posts of JS in Central Secretariat are entirely filled by C.St.S. and are not encadred for any service. The issue of encadrement of few posts of JSs in Central Secretariat for CSS was examined in the Second Cadre review also. It was decided that the posts of JSs in Central Secretariat should only be filled with C.St.S. and should not be encadred for CSS or for any other service. The Committee recommends that the same should be continued. The Committee, however, felt that there is a case for encadrement of posts dealing with general administration for CSS in other organizations including attached/subordinate offices. In this regard, a communication was sent to all the Departments to suggest posts which could be encadred at JS level in CSS in such organizations. However, no such proposal was received from any Department. The
Page 52
Committee recommends that posts at JS level outside Central Secretariat in other organizations including attached/subordinate offices could be encadred in CSS based on the proposals made by those Departments. These numbers would be in addition to the limit of 40 for JS in situ. A committee consisting of Secretary (Personnel), Establishment Officer and JS (AT\&A) should be authorized to examine and decide on such proposals. The Committee, however, does not recommend any increase in the strength of JS in-situ from 40.
| 40 | 40 | 40 |
|---|---|---|
| 40 | 40 | 40 |
| 40 | 40 | 40 |
| 40 | 40 | 40 |
(vi) Reserves in CSS
- The $2^{\text {nd }} C R C$ had recommended reserves in CSS taking note of the fact that a number of officers in CSS usually remained away on deputation, leave or training. A provisions has been made in CSS Rules, 2009 for 20\% deputation reserve, 3\% Leave Reserve and 1\% Training Reserve of the sanctioned strength of US and above level posts. The Rules provide that these reserves are over and above the sanctioned strength. 10.1. Provisions of Reserves as provided in CSS Rules has, however, never been operated so far perhaps for the reason that no methodology has been in place. As in other services, Reserve is to be operated at the entry grade. In the case of CSS too, assessment of the actual need for reserves and reporting of vacancies at the lowest level of Assistant should be done in advance. The Committee has accordingly deliberated the issue with reference to guidelines of CRD of DoPT. The OM dated 11.2.2013 of CRD, DoP\&T lays down the revised norms for reserves in the organized Group ‘A’ services: (i) Training Reserve – Not exceeding 1.5\% of SDP (ii) Leave Reserve – Not exceeding 1.5\% of SDP (iii) Deputation Reserve – Not exceeding5\% of SDP (iv) Probationary Reserve equal to period of probation (x) DR batch size 9.2 The CRD guidelines on probationary reserve may not be applicable in the case of CSS as the duration of training of DR Assistants is only for a period of 12 weeks as compared to 1-2 years in Group ‘A’ services. Therefore, reserves in CSS should
operate against Training, Long leave and Deputations only. Therefore the Committee recommends that the provisions of reserves which are already available in the CSS Rules may be modified to the extent of provisioning against deputation, training and long leave only and a reserve of $8 \%$ of the revised cadre strength of SO and above levels in CSS should be operated at Assistant grade to meet the shortfall and requirement of manpower across Ministries/Departments. The Committee also recommends that Ministries/Departments where the reserve may sometimes need to be attached would be authorized to draw salary. Other than the Assistant grade, reserves in CSS should not be maintained in any other grade. CSS Rules, 2009 should be amended accordingly.
Revised Cadre Strength
- In the foregoing paragraphs, the Committee has attempted to examine all the relevant issues to arrive at an optimum solution to the problem of meeting the functional needs to the Central Secretariat and also to harmonize it to the extent possible with the reasonable career expectations of the CSS. If the recommendations of the committee is finally accepted, the revised strength in various grades and changed hierarchical structure will be as under:
Table 8 : Proposed Revised Cadre Strength of CSS/CSCS
| Designation | Strength | Total | |
|---|---|---|---|
| Present | Proposed | ||
| LDC + UDC | $300+3700$ | $2000+2000$ * | $4000 *$ |
| (over the years) | |||
| Assistant | 6577 | 6577 | 6577 |
| SO | 3096 | $3096+463$ (Desks) | 3559 |
| US | 1538 | $1538+232$ (Desks) | 1770 |
| DS/Dir | 600 | 675 | 675 |
Note: * There are 3700 UDCs (regular + adhoc) and 300 LDCs. Out of the 3700 UDCs, DoPT proposes to give adhoc promotion to 1500 UDCs to Assistant grade immediately to fill up vacancies in the grade of Assistant. There are about 2000 physical vacancies in the grade of Assistant as on date. Approximately 500 more UDCs will get promoted by 2015, leaving only about 1700 UDCs who will also get promoted to Assistant grade over a period of time against the revised proposed promotion quota of $40 \%$. There will be no promotion to the UDC grade immediately as the newly recruited LDCs will be eligible for promotion after 8 years of residency service.
THIRD CADRE RESTRUCTURING OF CSS
Proposed Central Secretariat Pyramid
THIRD CADRE RESTRUCTURING OF CSS
Proposed Reporting Structure in Central Secretariat
Page 57
10.2 Financial Implication: Over the next few years, there would be large reduction in the numbers of UDCs, who would be promoted as Assistant. The number of DS/Dir, US and SO working on desk pattern would increase. Direct recruitment of LDCs and their promotion as UDC to fill the posts of LDC/UDC would take almost 10 years. Therefore, on net basis, there would be no additional expenditure as a result of cadre restructuring. Details are given in Annexure III.
Chapter 3
Fast Track Promotion in CSS : Replacement of LDCE
Limited Departmental Examination (LDCE) was conceptualized and implemented in the Central Secretariat with a view to affording an opportunity to meritorious officers to move up in their career through a fast track without waiting for the completion of residency in a grade. The Committee has deliberated in detail the existing pattern of LDCEs for various grades of CSCS and CSS in the backdrop of requirement for improvement in the noting & drafting skills of officers. It is also observed that in the combined SO LDCE 2009-11 conducted by UPSC for promotion from Assistant to SO grade most of the candidates, who applied, finally qualified also.
- The LDCE mechanism has been envisaged to provide a fast-track career to meritorious officials. The idea of holding LDCE and giving fast promotions to the successful candidates is to promote merit and the very objective stands defeated if the examination results in practically no elimination and pass percentage range above 90%.
-
UPSC and SSC have been holding Limited Departmental Competitive Examinations (LDCE) for promotion to SO level and UDC level respectively for quite some time. These Exams are Multiple Choice Questions (MCQ) based on various subjects that are relevant for CSS/CSCS officers. The main skill/aptitude required in CSS/CSCS is analytical abilities and writing skills. The present LDCEs do not test noting & drafting skills and there has been no systematic teaching system/course material available for these exams.
-
The Committee has considered other modes for providing fast-track career opportunities to the deserving officials of the Central Secretariat and contemplated linking the promotion of the officials to some academic courses which would provide for their skill enhancement and value addition. Towards that end, introduction of customized courses has been considered for providing fast track career progression to meritorious officials up to US grade in the CSS/CSCS who could complete the prescribed level courses and achieve certain level of proficiency in the examination conducted by IGNOU. The idea is to hold capacity building courses through IGNOU and using performance in these capacity building courses as a yardstick to providing fast track career opportunities.
-
At the concept level, the Committee is of view that there should be a 4-tier modules for such customized courses i.e. for (i) Promotion from LDC to UDC (ii) Promotion from UDC to Assistant Grade (iii) Promotion from Assistant Grade to SO
Page 59
Grade and (iv) Promotion from SO to US Grade. The qualification with a higher percentage by the participant officials in the relevant grades shall be compulsory. The inter-se seniority of top officials eligible for promotion through the fast track mode may also get altered as they will be interpolated with seniority quota officials based on their merit and position in the exam.
6. The Committee accordingly recommends that there should be customized courses on Public Policy as under:
Table 9: Customised courses for fast track promotion
| Level | For Promotion | Duration of course |
Residency required for promotion |
|
|---|---|---|---|---|
| From | To | |||
| Level I(UG level) | LDC | UDC | 2 years | A uniform of 5 years’ approved service in the lower grade |
| Level II(UG level) | UDC | Assistant | 1 year | |
| Level III(PG level) |
Assistant | SO | 2 years | |
| Level IV(PG level) |
SO | US | 1 year |
Under-Graduate Level courses (Level I \& II) on Public Policy
6.1 Courses should be of under-graduate level meant for LDCs who desire to get promoted to the grade of UDC and an advance version of the same for promotion from UDC to Assistant grade. There should be a minimum pass percentage as well as an honors percentage and those LDCs, who get honors marks would be eligible to get promotion on a fast track under the merit / departmental examination quota for UDC after completion of Level I and that for Assistant after completion of Level II. The Level I course would be of 2 years’ duration with four semesters and Level II would be of one year duration with two semesters.
Post Graduation level courses (Level III \& Level IV) on Public Policy
6.2 The level of the course should be of Post-Graduate level meant for Assistant of both Direct Recruits and Promotees and an advance version of the same for SOs. The Level III course would be of 2 years’ duration with four semesters and Level IV would be of one year duration with two semesters.
Course should have necessary papers of Law, Indian Economy, Constitution, 20B Administrative Law, Public Administration, Govt. Procedures and Manuals, Financial Management. Course should have a pass level and honors level and those Assistants, who pass the courses with honors would be eligible for promotion to SO and US grade under the Examination Quota.
- An Officer may enroll for course specified for the promotion grade without any condition of minimum eligibility service and also without any stipulation of number of attempts/ years for completion. Qualification with Honors degree i.e. achieving the prescribed higher percentage by the participant officials in the relevant grades would be compulsory for an official to be entitled for fast track career progression. However, mere qualification would not entitle him or her to be placed in a higher grade as this would be subject to the availability of vacancy in each of the grades in a particular year. The qualified officials in the order of merit would be treated under Exam Quota (EQ) and they would be considered for promotion along with the seniority quota officials in the ratio as below:
Table 10: Revised promotion ratio
| Level | Existing ratio between LDCE quota & seniority quota % | Proposed ratio between Exam quota & seniority quota % |
|---|---|---|
| From LDC to UDC | 25 : 75 | 50 : 50 |
| UDC to Assistant | (75DR) : 10LDCE : 15SQ | 50 : 50 (out of 40% promotion quota) |
| Assistant to SO | 50 : 50 | 25 : 75 |
| SO to US | 100% by promotion | 25 : 75 |
7.1 Notwithstanding the above, the vacancies remaining unfilled, if any, will be transferred to the seniority quota in the same year without any carry-forward to the subsequent year.
- There should be a fixed cost component to be borne by the DoPT as well as the variable cost components depending upon the number of applicants, to be borne also by DoPT. All expenses on customized courses including conduct of examination for employees of Central Secretariat could be met by DoPT.
Page 61
- Having examined the relevant aspects in broad, the Committee recommends that DoPT should process the matter so that customized Courses for fast track career progression for CSCS/CSS officials are in place and pending implementation of the Scheme, the existing arrangements shall not be disturbed.
Chapter 4
Revision of period of residency for promotion from one grade to the next higher grade in CSS/CSCS
The CSCS Rules and the CSS Rules 2009 provide a residency period or qualifying service in a grade as a qualification for promotion to the next grade. These residency requirements are specified for each grade and computed as approved service rendered in a grade. Residency requirements for promotion from one grade to the next higher grades are as under:
Table 11: Residency service for promotion
| From | To | Mode of Recruitment | Residency service (in terms of years of approved service in lower grade) |
|---|---|---|---|
| LDC | UDC | Promotion-SQ | 8 |
| LDCE | 5 | ||
| UDC | Assistant | Promotion-SQ | 10 |
| LDCE | 6 | ||
| Assistant | SO | Promotion-SQ | 8 |
| LDCE | 5 | ||
| SO | US | Promotion | 8 |
| US | DS | Promotion | 5 |
| DS | Dir | Promotion | 5 |
- After the 6th CPC, DOP&T has issued guidelines vide OM dated 24.3.2009 prescribing minimum qualifying service in various grade pay for promotion to the next higher grade pay. The minimum qualifying service prescribed in the aforesaid OM dated 24.3.2009 relates to the actual regular service, whereas there is concept of approved service in CSS, which is the basis for deciding eligibility for promotion. Irrespective of the actual date of joining, the approved service in a particular grade starts from 1st July of the Select List year (promotion/recruitment year) in which an officer has been included. Therefore, in case of CSS officers, regular or actual service in a grade is much less than their approved service and residency requirement has to be seen in this background.
THIRD CADRE RESTRUCTURING OF CSS
- The issue of residency service has been a very contentious one in CSS. Representations have been received for:
(i) revising the periods of residency for promotion from one grade to another in CSS as per the guidelines prescribed in DOP&T OM dated 24.3.2009;
(ii) revising the eligibility service for promotion from Assistant to SO’s grade through LDCE/SQ;
(iii) providing for a combined eligibility service of 10 years in the grades of US and DS with not less than 3 years in the grade of DS for promotion to the grade of Dir in the analogy of the position in CSSS.
3.1. CSCS Associations have also represented for revisiting the eligibility of service prescribed for promotion from UDC to Assistant Grade.
- The Committee has noted that the change in residency period, if any, for promotion to various grades of CSS would have to be considered keeping in view the functional competency required of officers to effectively handle the job. The residency or eligibility of service is the minimum required period that an official has to serve in a grade for his consideration for promotion to the next grade. The guidelines as contained in Estt (RR) OM dated 24.3.2009, however, make it amply clear that for placement in the next higher grade pay, the time period shown therein is the minimum years of qualifying service and hence it hardly needs any emphasis that the residency would vary from service to service depending upon various factors including, inter alia, the structure and size, hierarchical pyramid, level of maturity and experience required for holding a particular post or grade. The Committee has examined the issue of residency grade wise and its recommendations are given in the following paragraphs.
LDC to UDC grade
5.1. The Grade Pay of LDC is Rs.1900 and that of UDC is Rs.2400. The existing residency of 8 years through seniority quota is adequate and it also meets the minimum requirement as stipulated in the guidelines of Estt (RR).
UDC to Assistant Grade
5.2 CSS Regulations 2013 prescribe a residency requirement of 10 years for promotion from UDC to Assistant. Earlier, under CSS Rules, 1962, the residency for promotion to Assistant was 5 years. There is a demand for reducing residency under CSS Regulations 2013 also to 5 years.
Page 64
THIRD CADRE RESTRUCTURING OF CSS
5.2.1 The 6th CPC has fixed the Grade Pay of UDC as Rs.2400 and that of Assistant as Rs.4600. The minimum residency requirement as stipulated in the guidelines of Estt (RR) for moving from GP of Rs.2400 to Rs.4600 is 15 years. The GP of Assistant has gone up by higher percentage, as compared to UDC under 6th CPC. This has resulted in increased residency requirement of 15 years for promotion from UDC to Assistant.
5.2.2 The Associations have been raising this issue for the last 3-4 years when the CSS Regulations 2013 were being finalized. In recognition of the fact that residency of 15 years for promotion to Assistant is high, DoPT had, in relaxation of the residency requirement prescribed by the Establishment Division, had reduced it to 6 years through LDCE and 10 years through SQ for promotion from UDC to Assistant Grade under CSS Regulations 2013. However, the issue has already been examined in DOPT in detail and in pursuance thereof Regulations have been issued very recently stipulating the minimum requirement of 6 years for LDCE and 10 years through SQ for promotion from UDC to Assistant Grade. Further, the promotional delay in the grade of UDC is a temporary phenomenon and it is mainly attributable to SL 2003 which is unusually large and once this is exhausted, there would be no significant delay in the promotion of UDCs. The Committee accordingly feels that the residency prescribed for promotion from UDC to Assistant grade is already on the lower side than that prescribed under Estt (RR) guidelines. Therefore, committee has not recommended changing the existing provisions.
Assistant to SO and SO to US grades
5.3 Residency period for promotion to various grades of CSS will have to be considered keeping in view the functional competency required of officers of a grade to effectively handle the job. The guidelines as contained in Estt. (RR) OM dated 24.3.2009 make it explicitly clear that for placement in the next higher grade pay, the time period shown therein is the minimum years of qualifying service and hence it hardly needs to be emphasized that the residency will vary from service to service depending upon various factors including the structure and size, vacancies arising as also the level of maturity and experience required for holding a particular post or grade.
5.3.1 The residency requirement for promotion from Assistant to SO is 5 years for LDCE and 8 years under SQ. The residency requirement at this level was same in the CSS Rules 1962 also and was not changed after 6th CPC. The residency requirement prescribed by the Estt. Division at this level is 2 years. This is quite low, the reason being that GP at Assistant level has been increased more than that at SO level after the 6th CPC. Due to the concept of approved service, many Assistants of CSS are eligible
Page 65
for appearing for LDCE with less than 5 years of actual service. In the case of promotion through seniority mode, the data available shows that the time taken for promotion to the SO grade through seniority would continue to be around 10 years. Moreover, it is desirable that the officer gains adequate experience and maturity to shoulder the responsibilities of SO which are supervisory levels in the Secretariat. Having regard to all these facts, the Committee feels that the existing residency of 8 years for promotion from Assistant to SO Grade is appropriate and there is no need to change the same.
5.3.2 The residency requirement for promotion from SO to US under the CSS Regulations 2013 is 8 years. The residency requirement at this level was same in the CSS Rules 1962 also and was not changed after $6^{\text {th }}$ CPC. The residency requirement prescribed by the Estt. Division at this level is 6 years. Due to the concept of approved service, the regular services performed at SO level, that is the time taken for being eligible for promotion to US after getting the promotion as SO in practice, is less than the residency prescribed of 8 years. Presently, SOs are promoted as US after 10 years of approved service and is likely to continue to be 10 years. Moreover, it is desirable that the officer gains adequate experience and maturity to shoulder the responsibilities of US. Having regard to all these facts, the Committee feels that the existing residency of 8 years for promotion from SO to US is appropriate and there is no need to change the same.
US to DS Grade
5.4 The Committee has noted that the residency prescribed for promotion from US to DS Grade of CSS is 5 years which is as per the guidelines of Estt. (RR). The committee has examined the issue and it is observed that following the present residency period of 5 years for promotion from the grade of US to DS is practically not possible. As per the data available, it is taking about 10 years for promotion from US to DS grade. Under MACP scheme also period prescribed for promotion for financial upgradation from one grade to another is ten years. Therefore, the Committee does not recommend any change in the residency already prescribed for the US grade for promotion to the DS grade.
DS to Dir
5.5 The CSS Rules prescribe a residency of 5 years approved service in the grade of DS for promotion to the Dir Grade and this is as per guidelines of Estt (RR). Demands have been made from various quarters of CSS officers for change in residency requirement for promotion to Dir Grade from 5 years of approved service to ” 5 years approved service failing which a total combined service of 10 years at US \& DS levels of which there shall be a minimum of 3 years of service at DS level”, as similar provisions are already available to CSSS officers.
5.6 In CSS, as of now there are no eligible DSs with 5 years of approved service for being promoted to Dir Grade as the year 2008 was a no panel year for DS. The posts of DS \& Dir in CSS are inter-changeable, and presently the vacancies in the Dir are being operated in the DS Grade and most of the officers who presently are DS, are retiring in the DS grade without completing 5 years of approved service for promotion to the grade of Dir.
5.7 Having analyzed all these issues, the Committee recommends that the residency requirement for promotion to the grade of Dir should be modified to 5 years approved service failing which a total combined approved service of 10 years at US \& DS levels of which there shall be minimum regular service of 3 years at DS level. However, since the entire process of cadre review might take time, as a stand-alone case, this issue should be taken up separately. Powers of relaxation are in built in the CSS Rules and DoPT should process the case for approval of the competent authority for relaxation and granting promotion to eligible DSs on the basis of combined approved service in US and DS grade of 10 years with a stipulation of minimum 3 years of regular service in the DS grade. The suggested measure would address the major grievance/demand made from various quarters of CSS.
| 10 years | 10 years | 10 years | 10 years |
|---|---|---|---|
| 10 years | 10 years | 10 years | 10 years |
| 10 years | 10 years | 10 years | 10 years |
| 10 years | 10 years | 10 years | 10 years |
| 10 years | 10 years | 10 years | 10 years |
Chapter 5
Deputation of CSS Officers
In terms of Rule 5 (2) of CSS Rules, 2009 there is a deputation reserve of $20 \%$ of the posts of US and above level. This reserve is in addition to the authorized sanctioned strength. Accordingly, at any given point of time, upto a maximum of $20 \%$ of the officers could be allowed to be on deputation. Vacancies caused by deputation could be filled up by promotion of officers in the feeder grade to the extent of deputation vacancies. 2. As against a deputation reserve of $20 \%$, the number of CSS officers on deputation is very low as per the data as on 1.8.2013 as under:
Table 12: CSS Officers on deputation
| Designation | Sanctioned
strength | No. of officers on
deputation | \% officers on
deputation to
sanctioned strength |
| — | — | — | — |
| Dir\&
JS (in-situ) | 185 | 15 | $8 \%$ |
| DS | 446 | 10 | $2.2 \%$ |
| US | 1538 | 76 | $5 \%$ |
- Deputation of CSS Officers is regulated under the general guidelines issued by Establishment Division of DoPT. No separate guidelines exist for CSS Officers.
- Following are the reasons for low percentage of CSS officers on deputation: (i) there is a bar on officers proceeding on deputation after attaining 56 years of age and most of the promotee officers in DS/Dir grade are in this age bracket and hence they are not eligible to apply for deputation; (ii) in terms of guidelines issued by D/o. Pension \& Pensioners Welfare, vide their OM No.4/78/2006-P\&PW(D) dated 31.10.2007 (Annexure-IV), Central Govt. officers can be appointed in Central Autonomous Bodies (CABs) on immediate absorption basis only. If a post in a CAB is to be filled on deputation basis, the post is required to be exempted from the rule of immediate absorption. Such exemption is granted by D/o. P\&PW keeping the following guidelines in view:
216
(a) Posts requiring specialized personnel in connection with scientific research or development of technology;
(b) Posts in executive or senior management level i.e. post carrying a pay scale of not less than DS grade in CAB having very close inter-action with policies and programmes of the Government;
(c) Posts where the nature of the work requires employment of Government officers for security reasons or vigilance purposes;
(d) Posts in newly established / temporary organizations (upto a period of five years from the date of establishment).
(e) Posts limited in number particularly in specialized fields where creation of a regular cadre is not feasible;
In terms of the above guidelines, posts in executive or senior management level i.e. posts of and above the level of DS in CAB having very close interaction with policies and programmes of the Govt. are considered for exemption. In view of this, posts of US and below levels in CABs are, generally, not exempted to be filled on deputation basis. However, in case of newly created CABs, all the posts are exempted upto a period of 5 years. However, exemption is to be specifically obtained from D/o. P&PW before filling up of posts on deputation basis. The instructions issued by D/o. P&PW also provide that without exemption if an officer is appointed on deputation basis in a CAB, he is treated as having resigned from the Central Govt. and absorbed in the CAB. He will forego counting of past service for pensionary benefits.
(iii) Though deputation guidelines for AIS officers and other organized Group A/ B services have been liberalized inter-alia allowing deputation of officers to Autonomous Organizations whether under the control of the Govt. or not, CSS Officers are not allowed to go on deputation to CABs in the scenario of such posts not exempted under the rule of immediate absorption basis.
- Small number of CSS Officers on deputation is one of the reasons for lack of promotional avenues in the cadre as vacancies caused by deputation could be filled up by promotion. Liberalizing deputation avenues will have a positive effect of increasing the promotional prospects in the cadre.
-
Over a period of years, deputation guidelines have been liberalised by Establishment (RR) Division and by AIS Division. AIS Cadre Rules provide the following liberal deputation guidelines:
Page 69
8.1. Rule 6(1) – A cadre officer may, with the concurrence of the State Governments concerned and the Central Government, be deputed for service under the Central Government or another State Government or under a Company, association or body of individuals, whether incorporated or not, which is wholly or substantially owned or controlled by the Central Government or by another State Government*
Accordingly, AIS officers are allowed deputation to organisations controlled by the Govt. as under:
(i) Autonomous Institutions wholly or substantially funded or controlled by the Central Government.
(ii) Central/ State PSUs (subject to exemption from rule of immediate absorption basis)
(iii) Constitutional bodies
(iv) Statutory bodies set up by an Act of Parliament
(v) Non-permanent, non-statutory bodies with a specific term set up through executive orders/ notification by Central Govt. viz. Administrative Reforms Commission, Pay Commission, National Manufacturing Competitiveness Commission etc.
8.2. Rule 6(2)(i): A cadre officer may also be deputed for service under a Company, association or body of individuals, whether incorporated or not, which is wholly or substantially owned or controlled by a State Government, a Municipal Corporation or a Local Body, by the State Government on whose cadre she/he is borne*
Accordingly, an AIS Officer can be appointed on deputation to the following organisations:
(i) A municipal Corporation or a Local Body of the State
(ii) State Government PSUs
(iii) Training/Research/ Educational Institutions wholly or substantially funded or controlled by the State Government
(iv) Autonomous Institutions wholly or substantially funded or controlled by the State Government
THIRD CADRE RESTRUCTURING OF CSS
(v) A registered Trust or Society or Association or Body of Individuals wholly or substantially funded or controlled by the State Government
8.3. Rule 6(2)(ii): A cadre officer may also be deputed for service under an international organisation, an autonomous body not controlled by the Government, or a private body, by the Central Government in consultation with the State Government on whose cadre she/he is borne.
An AIS officer is allowed to proceed on deputation to following types of organizations not controlled by the Government:
(i) Registered Societies or Trusts or Foundations or non-profit organizations or NGOs or cooperatives;
(ii) Apex bodies of Industries and Commerce;
Provided that such autonomous or private bodies fulfil all four of the following criteria:
a. They are functionally autonomous of the Central and State Government;
b. They are not substantially funded by the Central and State Government;
c. The Central or State Governments do not have powers to give them directions; and
d. They are not companies registered under the Registration of Companies Act.
The above guidelines have been made applicable to members of the organized Group A and the Group B Services of the Central Government vide Establishment Division’s O.M. No. AB-14017/2/07-Estt(RR) dated 29.2.2008 (Annexure-V).
- Though DoPT’s OM dated 29.2.2008 has extended deputation to wide ranging organizations including Autonomous Bodies, Societies, NGOs etc. to all Group A and B services on the lines of AIS rules, the condition prescribed by D/o. P&PW prescribing that the posts in CABs should be exempted from immediate absorption before they are filled on deputation basis still continues. In view of this, CSS Officers are not allowed to go on deputation to CABs in the absence of such specific exemption. Deputation chances are mostly available in statutory bodies, Institutions, various authorities which are in the nature of CABs. These organizations concerned may not have obtained exemption to fill up the posts on deputation basis, and accordingly, the chances of CSS
Page 71
officers proceeding on deputation to such organizations are much restricted. It may, however, be noted that RRs of these organizations provide for filling up of posts on deputation basis inter-alia from Central Govt. officers. However, in view of the extant guidelines of D/o. P\&PW, requiring exemption for filling up of posts on deputation basis, CS.I Division, DoPT has not been able to give cadre clearance for deputation organizations such as CCI, NHAI, NMCC, Prasar Bharati, Brain Research Centre, AIIMS, RIMS etc.

Chapter 6
Encadrement of posts in the Autonomous Bodies, Statutory Bodies, Committees and Commissions in CSS
The authorised sanctioned strengths of various grades of CSS are specified in CSS Rules, 2009. After notification of these Rules, additions have been made to the sanctioned strength in all grades of CSS by way of encadrement into CSS in respect of newly created posts or hitherto ex-cadre posts, in the various Ministries/Departments, attached and subordinate offices. The reasons for encadreding ex-cadre posts into CSS were:
- (i) Most of ex-cadre posts such as Deputy Dir, Administrative Officer, Senior/Junior Analyst etc. were mostly occupied by CSS Officers;
- (ii) Encadreding them facilitated early filling up of the posts without advertisement and consultation with UPSC requiring less administrative expenditure and time to fill up them; and,
- (iii) It temporarily increased the cadre strength of CSS resulting in opening up promotional avenues.
- However, ex-cadre posts are encadred only at the request of concerned Ministry/Department and it also required repealing of the existing RRs for that post. Over a period of years, ex-cadre posts in various designations viz. Administrative Officer, Deputy Dir, Senior Analyst, Junior Analyst, Research Officer etc. have been encadred in CSS mainly up to the level of US. The number of posts (both newly created and ex-cadre) encadred in different grades of CSS since 2009 is as under:
| Grade | Encadred since 2009 |
|---|---|
| DS/Dir | 42 |
| US | 140 |
| SO | 96 |
| Assistant | 193 |
- It has been decided as a matter of policy that while posts created in Ministries/Departments with the approval of Cabinet/DoE would be encadred in CSS/CSSS/CSCS as the case may be, the posts created in organizations other than Ministries/Departments viz. autonomous/statutory institutions could be filled up by
Page 73
THIRD CADRE RESTRUCTURING OF CSS
officers of these services on deputation basis by operating deputation reserve. Accordingly, OM No.21/7/2007-CS.I(Desk) dated 2.2.2010, was issued by DOPT.
- However, in view of instructions issued by D/o. P&PW vide their OM dated 31.10.2007, there is restriction on officers proceeding on deputation to organizations in the nature of autonomous bodies without the posts being exempted from the rule of immediate absorption.
-
As discussed in the earlier chapter, CSS consists of grade upto Dir Level. In the Central Secretariat there are a total of 1200 DS/Dir of which 600 are CSS cadre posts and the remaining are mostly filled up under C.St.S. To address the problem of stagnation within the ranks of CSS especially in the grade of US, new options are to be found.
-
In the above backdrop, the present policy of not encadering posts in autonomous organizations which are in the nature of statutory bodies, organizations registered as Societies, Committees and Commissions set up under the executive orders/Notifications of the Government requires to be reviewed. The Committee therefore, recommends that posts in organizations which are in the nature of Autonomous Bodies may be encadred in the CSS at DS/Dir level. Enactement in such organizations at US and lower levels should not be done as CSS may itself not be able to meet that requirement. The Committee recommends that Ministries/Departments be asked to identify posts of generalist nature in the autonomous/ statutory organizations, Commissions/ Committees under their administrative control for encadering them in CSS at DS/Dir level.
-
However, service rendered in autonomous bodies is treated as Foreign Service as most of them are grantee institutions. In terms of Note 7 below Rule 33 of CCS Pension Rules, 1972 pay drawn by a Govt. servant while on foreign service shall not be treated as emoluments for the purpose of pension and the pay which he would have drawn under the Government had he not been on foreign service shall alone be treated as emoluments.
-
In view of this, it is required to be stipulated that if a CSS Officer is appointed against an encadred post in an autonomous body or similar organization, his service and the pay drawn on the post will be counted towards pension and other retirement benefits, in relaxation of the aforesaid Note 7 below Rule 33 of CCS (Pension) Rules. Further, to avoid any hardship if an officer retires from an encadred post in any CABs, his pension will be finalized by the Ministry/ Department under whose administrative control the organization concerned functions.
Page 74
| 1 | |||
|---|---|---|---|
| 2 | |||
| 3 | |||
| 4 | |||
| 5 | |||
| 6 | |||
| 7 | |||
| 8 | |||
| 9 | |||
| 10 | |||
| 11 | |||
| 12 | |||
| 13 | |||
| 14 | |||
| 15 | |||
| 16 | |||
| 17 | |||
| 18 | |||
| 19 | |||
| 20 | |||
| 21 | |||
| 22 | |||
| 23 | |||
| 24 | |||
| 25 | |||
| 26 | |||
| 27 | |||
| 28 | |||
| 29 | |||
| 30 | |||
| 31 | |||
| 32 | |||
| 33 | |||
| 34 | |||
| 35 | |||
| 36 | |||
| 37 | |||
| 38 | |||
| 39 | |||
| 40 | |||
| 41 | |||
| 42 | |||
| 43 | |||
| 44 | |||
| 45 | |||
| 46 | |||
| 47 | |||
| 48 | |||
| 49 | |||
| 50 | |||
| 51 | |||
| 52 | |||
| 53 | |||
| 54 | |||
| 55 | |||
| 56 | |||
| 57 | |||
| 58 | |||
| 59 | |||
| 60 | |||
| 61 | |||
| 62 | |||
| 63 | |||
| 64 | |||
| 65 | |||
| 66 | |||
| 67 | |||
| 68 | |||
| 69 | |||
| 70 | |||
| 71 | |||
| 72 | |||
| 73 | |||
| 74 | |||
| 75 | |||
| 76 | |||
| 77 | |||
| 78 | |||
| 79 | |||
| 80 | |||
| 81 | |||
| 82 | |||
| 83 | |||
| 84 | |||
| 85 | |||
| 86 | |||
| 87 | |||
| 88 | |||
| 89 | |||
| 90 | |||
| 91 | |||
| 92 | |||
| 93 | |||
| 94 | |||
| 95 | |||
| 96 | |||
| 97 | |||
| 98 | |||
| 99 | |||
| 100 | |||
| 101 | |||
| 102 | |||
| 103 | |||
| 104 | |||
| 105 | |||
| 106 | |||
| 107 | |||
| 108 | |||
| 109 | |||
| 110 | |||
| 111 | |||
| 112 | |||
| 113 | |||
| 114 | |||
| 115 | |||
| 116 | |||
| 117 | |||
| 118 | |||
| 119 | |||
| 120 | |||
| 121 | |||
| 122 | |||
| 123 | |||
| 124 | |||
| 125 | |||
| 126 | |||
| 127 | |||
| 128 | |||
| 129 | |||
| 130 | |||
| 131 | |||
| 132 | |||
| 133 | |||
| 134 | |||
| 135 | |||
| 136 | |||
| 137 | |||
| 138 | |||
| 139 | |||
| 140 | |||
| 141 | |||
| 142 | |||
| 143 | |||
| 144 | |||
| 145 | |||
| 146 | |||
| 147 | |||
| 148 | |||
| 149 | |||
| 150 | |||
| 151 | |||
| 152 | |||
| 153 | |||
| 154 | |||
| 155 | |||
| 156 | |||
| 157 | |||
| 158 | |||
| 159 | |||
| 160 | |||
| 161 | |||
| 162 | |||
| 163 | |||
| 164 | |||
| 165 | |||
| 166 | |||
| 167 | |||
| 168 | |||
| 169 | |||
| 170 | |||
| 171 | |||
| 172 | |||
| 173 | |||
| 174 | |||
| 175 | |||
| 176 | |||
| 177 | |||
| 178 | |||
| 179 | |||
| 180 | |||
| 181 | |||
| 182 | |||
| 183 | |||
| 184 | |||
| 185 | |||
| 186 | |||
| 187 | |||
| 188 | |||
| 189 | |||
| 190 | |||
| 191 | |||
| 192 | |||
| 193 | |||
| 194 | |||
| 195 | |||
| 196 | |||
| 197 | |||
| 198 | |||
| 199 | |||
| 200 | |||
| 201 | |||
| 202 | |||
| 203 | |||
| 204 | |||
| 205 | |||
| 206 | |||
| 207 | |||
| 208 | |||
| 209 | |||
| 210 | |||
| 211 | |||
| 212 | |||
| 213 | |||
| 214 | |||
| 215 | |||
| 216 | |||
| 217 | |||
| 218 | |||
| 219 | |||
| 220 | |||
| 221 | |||
| 222 | |||
| 223 | |||
| 224 | |||
| 225 | |||
| 226 | |||
| 227 | |||
| 228 | |||
| 229 | |||
| 230 | |||
| 231 | |||
| 232 | |||
| 233 | |||
| 234 | |||
| 235 | |||
| 236 | |||
| 237 | |||
| 238 | |||
| 239 | |||
| 240 | |||
| 241 | |||
| 242 | |||
| 243 | |||
| 244 | |||
| 245 | |||
| 246 | |||
| 247 | |||
| 248 | |||
| 249 | |||
| 250 | |||
| 251 | |||
| 252 | |||
| 253 | |||
| 254 | |||
| 255 | |||
| 256 | |||
| 257 | |||
| 258 | |||
| 259 | |||
| 260 | |||
| 261 | |||
| 262 | |||
| 263 | |||
| 264 | |||
| 265 | |||
| 266 | |||
| 267 | |||
| 268 | |||
| 269 | |||
| 270 | |||
| 271 | |||
| 272 | |||
| 273 | |||
| 274 | |||
| 275 | |||
| 276 | |||
| 277 | |||
| 278 | |||
| 279 | |||
| 280 | |||
| 281 | |||
| 282 | |||
| 283 | |||
| 284 | |||
| 285 | |||
| 286 | |||
| 287 | |||
| 288 | |||
| 289 | |||
| 290 | |||
| 291 | |||
| 292 | |||
| 293 | |||
| 294 | |||
| 295 | |||
| 296 | |||
| 297 | |||
| 298 | |||
| 299 | |||
| 300 | |||
| 301 | |||
| 302 | |||
| 303 | |||
| 304 | |||
| 305 | |||
| 306 | |||
| 307 | |||
| 308 | |||
| 309 | |||
| 310 | |||
| 311 | |||
| 312 | |||
| 313 | |||
| 314 | |||
| 315 | |||
| 316 | |||
| 317 | |||
| 318 | |||
| 319 | |||
| 320 | |||
| 321 | |||
| 322 | |||
| 323 | |||
| 324 | |||
| 325 | |||
| 326 | |||
| 327 | |||
| 328 | |||
| 329 | |||
| 330 | |||
| 331 | |||
| 332 | |||
| 333 | |||
| 334 | |||
| 335 | |||
| 336 | |||
| 337 | |||
| 338 | |||
| 339 | |||
| 340 | |||
| 341 | |||
| 342 | |||
| 343 | |||
| 344 | |||
| 345 | |||
| 346 | |||
| 347 | |||
| 348 | |||
| 349 | |||
| 350 | |||
| 351 | |||
| 352 | |||
| 353 | |||
| 354 | |||
| 355 | |||
| 356 | |||
| 357 | |||
| 358 | |||
| 359 | |||
| 360 | |||
| 361 | |||
| 362 | |||
| 363 | |||
| 364 | |||
| 365 | |||
| 366 | |||
| 367 | |||
| 368 | |||
| 369 | |||
| 370 | |||
| 371 | |||
| 372 | |||
| 373 | |||
| 374 | |||
| 375 | |||
| 376 | |||
| 377 | |||
| 378 | |||
| 379 | |||
| 380 | |||
| 381 | |||
| 382 | |||
| 383 | |||
| 384 | |||
| 385 | |||
| 386 | |||
| 387 | |||
| 388 | |||
| 389 | |||
| 390 | |||
| 391 | |||
| 392 | |||
| 393 | |||
| 394 | |||
| 395 | |||
| 396 | |||
| 397 | |||
| 398 | |||
| 399 | |||
| 400 | |||
| 401 | |||
| 402 | |||
| 403 | |||
| 404 | |||
| 405 | |||
| 406 | |||
| 407 | |||
| 408 | |||
| 409 | |||
| 410 | |||
| 411 | |||
| 412 | |||
| 413 | |||
| 414 | |||
| 415 | |||
| 416 | |||
| 417 | |||
| 418 | |||
| 419 | |||
| 420 | |||
| 421 | |||
| 422 | |||
| 423 | |||
| 424 | |||
| 425 | |||
| 426 | |||
| 427 | |||
| 428 | |||
| 429 | |||
| 430 | |||
| 431 | |||
| 432 | |||
| 433 | |||
| 434 | |||
| 435 | |||
| 436 | |||
| 437 | |||
| 438 | |||
| 439 | |||
| 440 | |||
| 441 | |||
| 442 | |||
| 443 | |||
| 444 | |||
| 445 | |||
| 446 | |||
| 447 | |||
| 448 | |||
| 449 | |||
| 450 | |||
| 451 | |||
| 452 | |||
| 453 | |||
| 454 | |||
| 455 | |||
| 456 | |||
| 457 | |||
| 458 | |||
| 459 | |||
| 460 | |||
| 461 | |||
| 462 | |||
| 463 | |||
| 464 | |||
| 465 | |||
| 466 | |||
| 467 | |||
| 468 | |||
| 469 | |||
| 470 | |||
| 471 | |||
| 472 | |||
| 473 | |||
| 474 | |||
| 475 | |||
| 476 | |||
| 477 | |||
| 478 | |||
| 479 | |||
| 480 | |||
| 481 | |||
| 482 | |||
| 483 | |||
| 484 | |||
| 485 | |||
| 486 | |||
| 487 | |||
| 488 | |||
| 489 | |||
| 490 | |||
| 491 | |||
| 492 | |||
| 493 | |||
| 494 | |||
| 495 | |||
| 496 | |||
| 497 | |||
| 498 | |||
| 499 | |||
| 500 | |||
| 501 | |||
| 502 | |||
| 503 | |||
| 504 | |||
| 505 | |||
| 506 | |||
| 507 | |||
| 508 | |||
| 509 | |||
| 510 | |||
| 511 | |||
| 512 | |||
| 513 | |||
| 514 | |||
| 515 | |||
| 516 | |||
| 517 | |||
| 518 | |||
| 519 | |||
| 520 | |||
| 521 | |||
| 522 | |||
| 523 | |||
| 524 | |||
| 525 | |||
| 526 | |||
| 527 | |||
| 528 | |||
| 529 | |||
| 530 | |||
| 531 | |||
| 532 | |||
| 533 | |||
| 534 | |||
| 535 | |||
| 536 | |||
| 537 | |||
| 538 | |||
| 539 | |||
| 540 | |||
| 541 | |||
| 542 | |||
| 543 | |||
| 544 | |||
| 545 | |||
| 546 | |||
| 547 | |||
| 548 | |||
| 549 | |||
| 550 | |||
| 551 | |||
| 552 | |||
| 553 | |||
| 554 | |||
| 555 | |||
| 556 | |||
| 557 | |||
| 558 | |||
| 559 | |||
| 560 | |||
| 561 | |||
| 562 | |||
| 563 | |||
| 564 | |||
| 565 | |||
| 566 | |||
| 567 | |||
| 568 | |||
| 569 | |||
| 570 | |||
| 571 | |||
| 572 | |||
| 573 | |||
| 574 | |||
| 575 | |||
| 576 | |||
| 577 | |||
| 578 | |||
| 579 | |||
| 580 | |||
| 581 | |||
| 582 | |||
| 583 | |||
| 584 | |||
| 585 | |||
| 586 | |||
| 587 | |||
| 588 | |||
| 589 | |||
| 590 | |||
| 591 | |||
| 592 | |||
| 593 | |||
| 594 | |||
| 595 | |||
| 596 | |||
| 597 | |||
| 598 | |||
| 599 | |||
| 599 | |||
| 600 | |||
| 601 | |||
| 602 | |||
| 603 | |||
| 604 | |||
| 605 | |||
| 606 | |||
| 607 | |||
| 608 | |||
| 609 | |||
| 610 | |||
| 611 | |||
| 612 | |||
| 613 | |||
| 614 | |||
| 615 | |||
| 616 | |||
| 617 | |||
| 618 | |||
| 619 | |||
| 620 | |||
| 621 | |||
| 622 | |||
| 623 | |||
| 624 | |||
| 625 | |||
| 626 | |||
| 627 | |||
| 628 | |||
| 629 | |||
| 630 | |||
| 631 | |||
| 632 | |||
| 633 | |||
| 634 | |||
| 635 | |||
| 636 | |||
| 637 | |||
| 638 | |||
| 639 | |||
| 640 | |||
| 641 | |||
| 642 | |||
| 643 | |||
| 644 | |||
| 645 | |||
| 646 | |||
| 647 | |||
| 648 | |||
| 649 | |||
| 650 | |||
| 651 | |||
| 652 | |||
| 653 | |||
| 654 | |||
| 655 | |||
| 656 | |||
| 657 | |||
| 658 | |||
| 659 | |||
| 660 | |||
| 661 | |||
| 662 | |||
| 663 | |||
| 664 | |||
| 665 | |||
| 666 | |||
| 667 | |||
| 668 | |||
| 669 | |||
| 670 | |||
| 671 | |||
| 672 | |||
| 673 | |||
| 674 | |||
| 675 | |||
| 676 | |||
| 677 | |||
| 678 | |||
| 679 | |||
| 680 | |||
| 681 | |||
| 682 | |||
| 683 | |||
| 684 | |||
| 685 | |||
| 686 | |||
| 687 | |||
| 688 | |||
| 689 | |||
| 690 | |||
| 691 | |||
| 692 | |||
| 693 | |||
| 694 | |||
| 695 | |||
| 696 | |||
| 697 | |||
| 698 | |||
| 699 | |||
| 700 | |||
| 701 | |||
| 702 | |||
| 703 | |||
| 704 | |||
| 705 | |||
| 706 | |||
| 707 | |||
| 708 | |||
| 709 | |||
| 710 | |||
| 711 | |||
| 712 | |||
| 713 | |||
| 714 | |||
| 715 | |||
| 716 | |||
| 717 | |||
| 718 | |||
| 719 | |||
| 720 | |||
| 721 | |||
| 722 | |||
| 723 | |||
| 724 | |||
| 725 | |||
| 726 | |||
| 727 | |||
| 728 | |||
| 729 | |||
| 730 | |||
| 731 | |||
| 732 | |||
| 733 | |||
| 734 | |||
| 735 | |||
| 736 | |||
| 737 | |||
| 738 | |||
| 739 | |||
| 740 | |||
| 741 | |||
| 742 | |||
| 743 | |||
| 744 | |||
| 745 | |||
| 746 | |||
| 747 | |||
| 748 | |||
| 749 | |||
| 750 | |||
| 751 | |||
| 752 | |||
| 753 | |||
| 754 | |||
| 755 | |||
| 757 | |||
| 758 | |||
| 759 | |||
| 760 | |||
| 761 | |||
| 762 | |||
| 762 | |||
| 763 | |||
| 764 | |||
| 765 | |||
| 767 | |||
| 767 | |||
| 768 | |||
| 769 | |||
| 770 | |||
| 771 | |||
| 772 | |||
| 773 | |||
| 774 | |||
| 775 | |||
| 776 | |||
| 777 | |||
| 777 | |||
| 778 | |||
| 779 | |||
| 780 | |||
| 781 | |||
| 782 | |||
| 783 | |||
| 784 | |||
| 785 | |||
| 786 | |||
| 787 | |||
| 788 | |||
| 789 | |||
| 790 | |||
| 791 | |||
| 792 | |||
| 793 | |||
| 794 | |||
| 795 | |||
| 796 | |||
| 797 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 791 | |||
| 799 | |||
| 7910 | |||
| 7911 | |||
| 7912 | |||
| 793 | |||
| 794 | |||
| 795 | |||
| 796 | |||
| 797 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 7913 | |||
| 799 | |||
| 7914 | |||
| 7915 | |||
| 799 | |||
| 7916 | |||
| 7917 | |||
| 7918 | |||
| 7919 | |||
| 7920 | |||
| 7921 | |||
| 7922 | |||
| 793 | |||
| 794 | |||
| 795 | |||
| 796 | |||
| 797 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 7910 | |||
| 7911 | |||
| 7912 | |||
| 7913 | |||
| 7914 | |||
| 7915 | |||
| 7917 | |||
| 7918 | |||
| 7919 | |||
| 7922 | |||
| 793 | |||
| 794 | |||
| 795 | |||
| 796 | |||
| 797 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 7910 | |||
| 7911 | |||
| 7912 | |||
| 793 | |||
| 794 | |||
| 797 | |||
| 797 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 7913 | |||
| 799 | |||
| 799 | |||
| 7914 | |||
| 7915 | |||
| 7916 | |||
| 7917 | |||
| 7918 | |||
| 7919 | |||
| 7920 | |||
| 7921 | |||
| 7922 | |||
| 793 | |||
| 793 | |||
| 794 | |||
| 794 | |||
| 795 | |||
| 796 | |||
| 797 | |||
| 797 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 7910 | |||
| 7911 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 7912 | |||
| 79 | |||
| 79 | |||
| 799 | |||
| 799 | |||
| 7913 | |||
| 7914 | |||
| 7915 | |||
| 7916 | |||
| 7917 | |||
| 7918 | |||
| 7919 | |||
| 7918 | |||
| 7919 | |||
| 7919 | |||
| 7919 | |||
| 7919 | |||
| 7919 | |||
| 7919 | |||
| 7920 | |||
| 7921 | |||
| 7922 | |||
| 793 | |||
| 793 | |||
| 793 | |||
| 793 | |||
| 794 | |||
| 794 | |||
| 794 | |||
| 794 | |||
| 794 | |||
| 794 | |||
| 795 | |||
| 795 | |||
| 796 | |||
| 796 | |||
| 796 | |||
| 797 | |||
| 797 | |||
| 797 | |||
| 798 | |||
| 798 | |||
| 798 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 799 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 | |||
| 79 |
THIRD CADRE RESTRUCTURING OF CSS
Chapter 7
Miscellaneous Issues
This chapter contains Committee deliberations and findings on some of the demands raised by the associations during their presentation before the members of the committee.
Grant of time scale/ personal upgradation to US
- In terms of CSS Rules 2009, five years of approved service is required as the eligibility service for promotion from US to DS grade and thus USs up to SL-2008 are eligible for promotion to DS grade. It was noted that USs figuring in SLs 2003 & 2004 would get promoted to DS grade in the coming few months and there were some delays in promotion as all the promotions were vacancy based. The Committee considered whether it was feasible to grant the pay of the promotional post by placing the USs in higher scale without change of their designation and without creating new posts in the higher grade.
2.1 Grant of higher scale without change in designation etc. will create more problems than it will address. The present grade pay of US is 6600 and that of DS is 7600. There is no grade pay in between these two Grade Pays therefore if USs are to be granted higher grade pay on completion of certain number of years in service as a precursor to their promotion to DS grade, this may be in-situ promotion in disguise. USs with a higher grade pay might not be comfortable reporting to DS/Dir. In the past in-situ promotion had been given at US and DS levels to address to the problem to stagnation. In the present context when promotional prospects of CSS officers have improved significantly, there is no rational for placing US in the higher pay scale before their actual promotion. Moreover, MACP (Modified Assured Career Progression) Scheme is already in place to ensure at least three financial up-gradations in the absence of timely promotion.
Non-Functional Selection Grade (NFSG) in PB-4 (GP of Rs. 8700) in CSS
- Demands have been raised from various quarters for grant of NFSG in PB-4 to CSS officers on the basis of combined service in the US and DS grades.
Page 76
3.1 The demand for Dir grade in NFSG is mainly because of it being in the PB-4, and pay difference between DS and Dir. is considerable. However, it has to be considered whether grant of PB-4 (GP 8700) is possible within the frame of existing rules and instructions.
3.2 Non Functional Selection Grade was not agreed to by the 1st CRC on the ground that CSS was not a group ‘A’ service. There has been no change in the status of CSS. CSS officers are inducted into Group ‘A’ on their promotion to US grade. In the hierarchical structure of CSS, Dir is a promotion post in PB-4 with a Grade Pay Rs.8700/-. A DS (a post in PB-3 with GP of Rs.7600/-) with 5 years of approved service is eligible for promotion to the grade of Dir.
3.3 Due to non-availability of eligible DS with 5 years of service, the vacancies in the grade of Dir are operated at the level of DS. As against the ceiling of 220 post of Dir, presently there are approximately 125 officers appointed as Dir and the remaining posts are being operated at the level of DS. NFSG could only be useful if adequate number of Dir posts are not there for eligible DSs. This is not the case in CSS.
3.4 The committee has not considered the demand for NFSG to be actionable.
Lateral Entry of CSSS into CSS
- It has been decided that the CSS Cadre Re-structuring Committee would examine the matter and make suitable recommendations. The Committee accordingly has taken up the issue of lateral entry of Stenographers Grade ‘C’/PA in the SOs Grade of CSS by way of Departmental Examination (LDCE).
4.1. It is noted that there has been a series of Court cases on the issue of lateral entry of CSSS into CSS in addition to various references received in DoP\&T from the MPs/VIPs.
4.2. The issue of lateral entry was examined by the 1st Committee on Cadre Restructuring of CSS also and in their report, it was inter-alia, observed that it was unfair to the CSS to allow lateral entry of CSSS officers into CSS. It recommended discontinuation of the lateral entry at both SO and US level. The decision on the recommendations of the first CRC of CSS on the stoppage of lateral entry of CSSS at both the SO and US level was, however, deferred by the Cabinet in October, 2003.
Subsequently, while deciding on the restructuring of CSSS in the year 2005, the Cabinet decided:-
(i) To discontinue the lateral entry of CSSS officers into CSS at the level of US of CSS.
(ii) To allow only those steno Grade ‘C’ who were graduates to participate in LDCE for SO Grade.
4.3. The order for discontinuation of lateral entry of CSSS into CSS at US level was issued by DoP&T on 29.7.2005 pursuant to which there were a spate of court cases filed by CSSS officials.
4.4. All the connected issues linked with lateral entry of CSSS into CSS in the SO’s Grade were examined by DoP&T and it was decided that the lateral entry of CSSS officers, who are Graduate, into CSS in the SO Grade as allowed by the Cabinet should be implemented. The above decision taken on 21.9.2010 was finally incorporated while notifying the Central Secretariat Services SOs’ Grade (LDCE) Regulations, 2010. The Regulations thus notified by the DoPT allowed the eligible graduates Steno Grade ‘C’ to appear in the SO LDCE.
4.5. The Committee has thus noted that the issue relating to lateral entry of Stenographers Grade ‘C’ into the CSS in the SO Grade stands settled and hence there is no reason in re-opening the issues as has been demanded by some of the CSSS associations in their representations. In another chapter, the Committee has recommended for replacing the existing LDCE system in various grades of CSCS and CSS with qualification in the customized courses with an aim to provide fast-track career progression to meritorious officials within a ceiling of 25% of the vacancies occurring in a particular year. The existing provision of allowing graduate Stenographers grade ‘C’/PA of CSSS with 5 years of approved service to compete for SOs’ LDCE should be replaced with the affording the Graduate Steno grade ‘C’/PA the opportunity to qualify the customized course on the same terms and conditions i.e. they should be graduate and they should have rendered 5 years of approved service as Steno grade ‘C’ for fast track career progression for 25% of vacancies arising in the SO grade. In the revised scenario, both for Assistant and Stenographer grade ‘C’/PA, an interpolated list of successful Assistants and PAs based on their performance in the customized course will have to be prepared which will be the basis of providing fast track promotion to the candidates to the extent of 25% vacancies in a year in the grade of SO. The necessary amendments in the CSS rules will have to be effected accordingly.
Page 78
THIRD CADRE RESTRUCTURING OF CSS
226
| 1 | 2 | 3 | 4 |
| 5 | 6 | 7 | 8 |
| 10 | 11 | 12 | 13 |
| 15 | 16 | 17 | 18 |
| 20 | 21 | 22 | 23 |
| 25 | 26 | 27 | 28 |
| 30 | 31 | 32 | 33 |
| 35 | 36 | 37 | 38 |
In-situ promotion to DS grade
- In another chapter, the Committee has discussed the history of in-situ promotions in the CSS. The 1st CRC had inter alia observed that no separate scheme of in-situ promotions could be devised for the Central Secretariat employees due to its wider legal and financial implications and these temporary measures could not be institutionalized. It was in this backdrop that in-situ promotions in future were denied by the 1st Cadre Review Committee.
- The present Committee has deliberated the issue but does not find any merit in the demands for in-situ promotion which is an adhoc measure to the grade of DS on the basis of combined service in SO and US grade, in the light of three cadre restructuring exercises and improved promotional prospects.
Rotational Transfer Policy for CSS Officers
- There have been mixed views on transfers of CSS officer from one Ministry to another. To some it is avoidable to protect institutional memory and to some it is necessary to prevent forming of vested interests. The pros and cons of the issue have been examined and the committee observes that normally an officer should be posted out of cadre unit/Ministry/Department only on promotion and the existing maximum tenure policy for CSS should be revised as under: (i) The combined tenure of Assistant/SO in a particular Ministry/Department should be revised to 10 years as against the existing 7 years. (ii) The tenure of US should be raised to 7 years from the existing 5 years. (iii) There would be no change for the maximum tenure at DS/Dir level so as to keep parity with those coming under C.St.S. (iv) Exemption from RTP in respect of officials retiring within 2 years should continue in respect of all the grades of CSS. However, Assistants
THIRD CADRE RESTRUCTURING OF CSS
promoted from LDC grade be exempted from rotation if they are within 5 years of their retirement.
(v) DoPT shall consider the requests of Ministries/Departments for retention of individual officers covered under RTP on case-to-case basis if received with the approval of Secretary of the Ministry/Department. However, retention in such cases should not exceed 2 years.
6.1 However, the Committee observes that in matters of rotation and transfers no fixed rules could be framed and any officer could be rotated any time due to administrative exigencies in public interest and on the request of officers on case-to-case basis.
Training of promotee UDCs and promotee Assistants
7.1. There is no mandatory training programme for promotion to the grade of UDC. The mandatory training programme for CSS starts from Level ‘A’ for promotion from UDC to Assistant. Out of 4500 Assistants in position, more than 3000 are promoted from seniority quota and there are about 3700 UDCs and most of them have not been given any training. It is recommended that training need of these members of service should be more effectively addressed both for domain rules & knowledge and noting & drafting skills.
7.2. The primary mandate of ISTM is to train officers belonging to the three Central Secretariat Services. However, the sheer number of promotee officers at UDC/Assistant levels which account for more than 6000, any effective training by ISTM is not practically possible. It is further felt that imparting a general rule based training will not help in bringing the required change. For better drafting skills, an officer not only should have good language skills but also good domain knowledge. In view of this, any effective training should impart both techniques of good writing skills as well as core knowledge of the subject an officer was assigned with. As Ministries/Departments deal with varied businesses, the Committee feels that Ministries/Departments are better placed in imparting training on the core areas allocated to them while simultaneously providing training on better noting and drafting. Ministries/Departments should therefore, be asked to arrange periodic training to its staff promoted to the grades of UDC/Assistant. Periodicity of training may be decided by the concerned Ministries; each of such training could be of 1-3 weeks in duration. ISTM should prepare a training module and develop a pool of trainers from the serving officers, retired officers and by inviting outside experts. The expenditure on this account may be met from their allocated budget.
Page 80
Annexures
| Annexure
No. | Description | Page No. |
| — | — | — |
| I. | DoPT Order No. 19/2/2013-CS.I (P) dated 25.4.2013
for the constitution of the Cadre Review Committee of
CSS | 82 |
| II. | List of Associations | 83 |
| III. | Financial implication | $84-85$ |
| IV. | D/o P \&PW’s OM No. 4/78/2006- P\&PW(D) dated
31.10.2007 prohibiting deputation in Central
Autonomous Organisations | $86-89$ |
| V. | DoPT’s O.M. No.AB-14017/2/07-Estt(RR) dated
29.2.2006 – Consolidated guidelines on deputation/
foreign service for organized Group A and B services | $90-97$ |
| VI. | Grade wise stagnation scenario in various grades of
CSS | $98-100$ |
THIRD CADRE RESTRUCTURING OF CSS
ANNEXURE I
No 19/2/2013-CS-I(P)
Government of India
Ministry of Personnel, Public grievances and Pensions
(Department of Personnel & Training)
Lok Nayak Bhawan, New Delhi – 110003
April 25, 2013
ORDER
Subject: Constitution of a Committee for Cadre Restructuring of the Central Secretariat Service (CSS).
A Committee for cadre restructuring of the Central Secretariat Service (CSS) with the following composition and terms of reference is constituted:
Composition:
| (i) | Establishment Officer & Additional Secretary, DoP&T | Chairman |
|---|---|---|
| (ii) | Joint Secretary (CS), DoP&T | Member |
| (iii) | Joint Secretary (Pers), D/o Expenditure | Member |
| (iv) | Director (CS-I), DoP&T | Member-Secretary |
Terms of Reference:
- (a) To review the structure of CSS cadre, along with the feeder cadre, so as to harmonise the functional needs with the legitimate career expectations of its members.
- (b) To assess the magnitude of stagnation in various grades of CSS and suggest remedial measures – both short-term and long-term, as to reduce promotional blocks and at the same time prevent gaps from building up.
- (c) To suggest measures to enhance the effectiveness of service and capacity building of its members.
- (d) To take into view the suggestions of the stakeholders, viz. participating Ministries, Associations and members of the service for cadre review.
- (e) To examine any issue as referred to it by the cadre controlling authority of CSS and Central Secretariat Clerical Service (CSCS).
The secretarial services to the Committee would be provided by the CS Division.
This has the approval of the Hon’ble Minister of State for Personnel.
(Parminder Singh)
Under Secretary to the Government of India
Tel. 24642705
Copy to:
- Establishment Officer & Additional Secretary, DoP&T, North Block, New Delhi
- Joint Secretary (CS), DoP&T, North Block, New Delhi
- Joint Secretary (Pers), D/o Expenditure, North Block, New Delhi
- Director (CS-I), DoP&T, Lok Nayak Bhawan, New Delhi
- Director (CS-II) and all Under Secretaries in CS-I & CS-II Divisions
- DoP&T website
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.01 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.01 \, \text{g}}{18.01 \, \text{g/mol}} = 18.01 \, \text{g/mol}$$
- Mass to Moles:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 18.01 g of 1 mole of $$H_2O$$.
- $$m = 18.01 \, \text{g/mol} = 18.01 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- P: Pressure (atm)
- V: Volume (L)
- n: Number of moles (mol)
- R: Ideal gas constant (0.0821 L·atm/mol·K)
- T: Temperature (K)
- n: Number of moles (mol)
- R: Ideal gas constant (0.0821 L·atm/mol·K)
- T: Temperature (K)
- n: Number of moles of $$H_2O$$ at constant temperature
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- V: Volume (L)
- P: Pressure (atm)
- V: Temperature (K)
- n: Number of moles (mol)
- R: Ideal gas constant (0.0821 L·atm/mol·K)
- T: Temperature (K)
- n: Number of moles of $$H_2O$$ at constant temperature
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- q: Heat transferred at constant pressure
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – \Delta H_0$$
- Equation: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
Hess’s Law1
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction1}} = \Delta H – \Delta H_0$$
- Equation: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- E: Cell potential
- E°: Standard cell potential
- R: Ideal gas constant
- T: Temperature (K)
- n: Number of moles of electrons transferred
- F: Faraday constant (96,485 C/mol)
- Q: Reaction quotient
Annexure-II
List of Associations
(i) CSS Group A Officers’ Association
(ii) CSS Forum
(iii) CSS (DR Gazetted) Association
(iv) Central Secretariat Assistants’ Association
(v) Central Secretariat Clerical Service Association
(vi) Central Secretariat Non-gazetted Employees Union
(vii) CSSS Gazetted Officers’ Association
(viii) Central Secretariat Stenographers’ Service Association (Group A)
(ix) Central Secretariat Stenographers’ Service Association
1. Introduction
1.1. Background
The study of quantum mechanics has revolutionized our understanding of the microscopic world. It provides a framework for describing the behavior of particles at atomic and subatomic scales. One of the key principles in quantum mechanics is the wave-particle duality, which states that particles can exhibit both wave-like and particle-like properties.
1.2. Objectives
The primary objective of this research is to explore the interaction between light and matter at the quantum level. Specifically, we aim to:
- Investigate the interaction between light and matter at the quantum level.
- Analyze the interaction between light and matter at the quantum level.
- Develop a quantum theory that uses the wave-particle duality principle to study the interaction between light and matter.
2. Literature Review
2.1. Quantum Mechanics
Quantum mechanics is a fundamental theory in quantum mechanics that describes the behavior of particles at atomic and subatomic scales. It provides a framework for describing the behavior of particles at atomic and subatomic scales. The principles of quantum mechanics are fundamental to modern physics, with key concepts such as wave-particle duality, wave-like nature, and particle-like properties.
2.2. Quantum Mechanics of Matter
The quantum mechanics of matter is fundamental to understanding the behavior of particles at atomic and subatomic scales. It provides a framework for describing the behavior of particles at atomic and subatomic scales. The principles of quantum mechanics are fundamental to modern physics, with key concepts such as wave-particle duality, wave-like nature, and particle-like properties.
3. Methodology
3.1. Experimental Setup
The experimental setup involved a laser source with a laser source. The laser source was used to generate a laser source with a laser source. The laser source was used to generate a laser source with a laser source. The laser source was used to generate a laser source with a laser source. The data was analyzed using the interactuarial analysis method.
3.2. Data Collection
Data was collected over a period of two years. The data was analyzed using the interactuarial analysis method. The data was analyzed using the interactuarial analysis method.
4. Results and Discussion
4.1. Observations
The experimental results revealed that the light can exhibit both wave-like and particle-like properties. The results indicate that the light can exhibit both wave-like and particle-like properties. The results support the theoretical predictions of quantum mechanics.
4.2. Observations and Analysis
The experimental results showed that the light can exhibit both wave-like and particle-like properties. The results indicate that the light can exhibit both wave-like and particle-like properties. The results support the theoretical predictions of quantum mechanics.
5. Conclusion
The study of quantum mechanics has transformed our understanding of the microscopic world and has revolutionized our understanding of the microscopic world. The results support the theoretical predictions of quantum mechanics and highlight the importance of quantum mechanics in modern physics.
Annexure-III Financial Implication of proposed Increase of posts in Cadre Review of CSS
| Designation | Sanctioned strength | Diff. | Pay Band | Mean pay | Grade Pay | DA@90% | Total | Financial implication per month |
|---|---|---|---|---|---|---|---|---|
| Existing | Proposed | |||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 (7+8) x90% |
| JS | 40 | 40 | 0 | PB-4 | 37400-67000 | 52200 | 10000 | 55980 |
| Dir | 220 | 220 | 0 | PB-4 | 37400-67000 | 52200 | 8700 | 54810 |
| DS | 340 | 415 | 75 | PB-3 | 15600-39100 | 27350 | 7600 | 31455 |
| US | 1538 | 1770 | 232 | PB-3 | 15600-39100 | 27350 | 6600 | 30555 |
| SO | 3096 | 3559 | 463 | PB-2 | 9300-34800 | 22050 | 4800 | 24165 |
| Asst | 6577 | 6577 | 0 | PB-2 | 9300-34800 | 22050 | 4600 | 23985 |
| UDC | 3700 | 1700 | -2000 | PB-1 | 5200-20200 | 12700 | 2400 | 13590 |
| LDC | 300 | 550 | 250 | PB-1 | 5200-20200 | 12700 | 1900 | 13140 |
| Total | 15811 | 14831 | ||||||
| Total financial implication per annum (-6879520 X 12) |
-82554240
Page 84
.
THIRD CADRE RESTRUCTURING OF CSS
Explanatory Note on Financial Implications
Creation of Posts in DS, US and SO grade of CSS: If the mechanism as recommended by the Committee is followed over next one and half year, and the posts are created following the usual procedure with due approval of Department of Expenditure and DoPT, the financial implications would be approx. Rs. 52.26 cr. per annum.
Assistant grade: No financial implications involved.
UDC grade: There is no recommendation as to any increase in the UDC and the existing lot of 3700 UDCs shall be reduced by 1700 as 1500 UDCs are to be promoted immediately as ad hoc Assistant and the another 500 UDCs would be promoted by the year 2015 and remaining strength of UDCs would be about 1700 only. Thus within a short period, 2000 UDCs will be reduced without any replacement being proposed by the Committee and there will be no increase in the grade of UDC for another 8 years when newly recruited DR LDCs will be eligible for promotion. Thus there is a saving of approx. Rs. 68.85 cr. per annum.
LDC grade: The financial implications involved in the intake of 250 DR LDCs would be Rs. 8.32 cr. per annum and an increase of equal amount in subsequent years.
Therefore, if proposal of CRC are accepted in its entirety, there would be an immediate saving of approx. Rs. 8.25 cr. This is primarily due to depletion in the ranks of UDC, which would be replaced with higher grade posts.
Page 85
Chemistry 101: Introduction to Chemical Bonding
1. Introduction
Chemical bonding is the process by which atoms combine to form molecules. It is a fundamental concept in chemistry that has led to the development of various types of chemical bonds. In this section, we will explore the fundamental principles of chemical bonding, including the basic principles of chemical bonding, the basic principles of chemical bonding, and the fundamental principles of chemical bonding.
2. Types of Chemical Bonds
2.1 Ionic Bonds
Ionic bonds are formed between metals and nonmetals. The metal atom is the most polar bond in the chemical bond. The metal atom is notívil in polar bonds, so it is a “sea of electrons” that can be converted into electrons. The electron pair is the most polar bond in the chemical bond, which is the electron pair in the metal atom.
2.2 Covalent Bonds
Covalent bonds are formed between nonmetals. The nonmetal atom is the most polar bond in the chemical bond. The nonmetal atom is the most polar bond in the chemical bond, which is the electron pair in the metal atom.
2.3 Dimm’s Bond
Dimm’s bond is the bond between atoms that combine to form molecules. The atoms combine to form molecules, which is the bond between atoms that combine to form molecules. The bonds are the same, but the bond is different.
3. Properties of Chemical Bonds
3.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
3.2 Bond Energy
The bond energy is the energy required to break a chemical bond. It is a measure of the bond energy of a chemical bond. The bond energy is the energy required to break a chemical bond.
3.3 Bond Energy
The bond energy is the energy required to break a chemical bond. It is a measure of the bond energy of a molecule. The bond energy is the energy required to break a chemical bond.
4. Properties of Chemical Bonds
4.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
4.2 Bond Energy
The bond energy is the energy required to break a chemical bond. The bond energy is the energy required to break a chemical bond.
5. Properties of Chemical Bonds
5.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
5.2 Bond Energy
The bond energy is the energy required to break a chemical bond. The bond energy is the energy required to break a chemical bond.
6. Properties of Chemical Bonds
6.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
6.2 Bond Energy
The bond energy is the energy required to break a chemical bond. The bond energy is the energy required to break a chemical bond.
7. Properties of Chemical Bonds
7.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
7.2 Bond Energy
The bond energy is the energy required to break a chemical bond. The bond energy is the energy required to break a chemical bond.
8. Properties of Chemical Bonds
8.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
8.2 Bond Energy
The bond energy is the energy required to break a chemical bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
9. Properties of Chemical Bonds
9.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
9.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
10. Properties of Chemical Bonds
10.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
10.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
11. Properties of Chemical Bonds
11.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
11.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
12. Properties of Chemical Bonds
12.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
12.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
13. Properties of Chemical Bonds
13.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
13.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
14. Properties of Chemical Bonds
14.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
14.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
15. Properties of Chemical Bonds
15.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
15.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
16. Properties of Chemical Bonds
16.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
16.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
17. Properties of Chemical Bonds
17.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
17.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
18. Properties of Chemical Bonds
18.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
18.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
19. Properties of Chemical Bonds
19.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
19.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
20. Properties of Chemical Bonds
20.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
20.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
21. Properties of Chemical Bonds
21.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond. The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
21.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
22. Properties of Chemical Bonds
22.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
22.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
23. Properties of Chemical Bonds
23.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
23.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
24. Properties of Chemical Bonds
24.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
24.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
25. Properties of Chemical Bonds
25.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
25.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
26. Properties of Chemical Bonds
26.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
26.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
27. Properties of Chemical Bonds
27.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
27.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
28. Properties of Chemical Bonds
28.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
28.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
29. Properties of Chemical Bonds
29.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
29.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
30. Properties of Chemical Bonds
30.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
30.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
31. Properties of Chemical Bonds
31.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
31.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
32. Properties of Chemical Bonds
32.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
32.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
33. Properties of Chemical Bonds
33.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
33.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
34. Properties of Chemical Bonds
34.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
34.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
35. Properties of Chemical Bonds
35.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
35.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
36. Properties of Chemical Bonds
36.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
36.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
37. Properties of Chemical Bonds
37.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
37.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
38. Properties of Chemical Bonds
38.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
38.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
39. Properties of Chemical Bonds
39.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
39.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
40. Properties of Chemical Bonds
40.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
40.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
41. Properties of Chemical Bonds
41.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
41.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
42. Properties of Chemical Bonds
42.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
42.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
43. Properties of Chemical Bonds
43.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
43.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
44. Properties of Chemical Bonds
44.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
44.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
45. Properties of Chemical Bonds
45.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
45.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
46. Properties of Chemical Bonds
46.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
46.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
47. Properties of Chemical Bonds
47.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
47.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
48. Properties of Chemical Bonds
48.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
48.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
50. Properties of Chemical Bonds
50.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
50.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
51. Properties of Chemical Bonds
51.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
51.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
52. Properties of Chemical Bonds
52.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
52.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
53. Properties of Chemical Bonds
53.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
53.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
54. Properties of Chemical Bonds
54.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
54.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
55. Properties of Chemical Bonds
55.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
55.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
56. Properties of Chemical Bonds
56.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
56.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
57. Properties of Chemical Bonds
57.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
57.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
58. Properties of Chemical Bonds
58.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
58.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
59. Properties of Chemical Bonds
59.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
59.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
60. Properties of Chemical Bonds
60.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
60.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
61. Properties of Chemical Bonds
61.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
61.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
62. Properties of Chemical Bonds
62.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
62.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
63. Properties of Chemical Bonds
63.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
63.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
64. Properties of Chemical Bonds
64.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
64.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
65. Properties of Chemical Bonds
65.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
65.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
66. Properties of Chemical Bonds
66.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
66.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
67. Properties of Chemical Bonds
67.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
67.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
68. Properties of Chemical Bonds
68.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
68.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
69. Properties of Chemical Bonds
69.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
69.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
70. Properties of Chemical Bonds
70.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
70.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
71. Properties of Chemical Bonds
71.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
71.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
72. Properties of Chemical Bonds
72.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
72.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
73. Properties of Chemical Bonds
73.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
73.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
74. Properties of Chemical Bonds
74.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
74.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
75. Properties of Chemical Bonds
75.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
75.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
76. Properties of Chemical Bonds
76.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
76.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
77. Properties of Chemical Bonds
77.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
77.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
78. Properties of Chemical Bonds
78.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
78.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
79. Properties of Chemical Bonds
79.1 Bond Length
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
79.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
80.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
81. Properties of Chemical Bonds
81.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
81.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
82.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
83.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
84.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
85.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
86.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
88.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
89.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
81. Properties of Chemical Bonds
81.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
81.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
81.3 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
82.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
83.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
84.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
85.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
86.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
88.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
89.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
82.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
83.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
84.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
85.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
86.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
88.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
89.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
82.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
83.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
84.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
85.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
86.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
88.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
89.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
82.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
83.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
84.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
85.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85.2 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
86.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
87. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
85. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
86. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
88. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
89. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
810 Bond Energy
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
82. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
83. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
84. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
**80. Properties of Chemical Bonds
**87.1 Bond Energy
The bond length is the distance between the metal atoms of the bond and the nonmetal atoms of the bond.
ANNEYURE. III
No.4/78/2006-P&PW (D)
Government of India
Ministry of Personnel, Public Grievances & Pensions
(Department of Pension & Pensioners’ Welfare)
3rd Floor, Lok Nayak Bhawan
New Delhi-110 003, Dated thest October, 2007
OFFICE MEMORANDUM
Subject:- Deputation of Central Government Servants to posts in Central Autonomous Bodies – Review of Policy.
The undersigned is directed to invite attention to this Department’s O.M. No. 4(12)/85-P&PW dated 31.3.87 stipulating, inter-alia, that appointment of Government servants in the Central autonomous bodies shall be on immediate absorption basis only as in the case of Central public sector undertakings and instructions contained in the Dept. of Pension & PW O.M. No. 4/42/87-P&PW dated 19.4.88 followed by O.M. of even number dated 29.1.91 and O.M. No. 4/10/2005-P&PW dated 5.12.2005 which provide for relaxation of the Rule of immediate absorption in respect of certain categories of posts in autonomous bodies. The matter has been reviewed by the Govt. and it has been decided that the instructions providing for appointment on immediate absorption basis should continue to be followed. The consolidated instruction superseding all earlier orders in the subject are as under. However in respect of the following categories of post in Central Autonomous Bodies (CABs) exemptions may be permitted:-
(a) Posts requiring specialised personnel in connection with scientific research or development of technology.
(b) Posts in executive or senior management level i.e. post carrying a pay scale of not less than (Rs. 12000-375-16500) in CAB having very close inter-action with policies and programmes of the Government.
(c) Posts where the nature of the work requires employment of Government officers for security reasons or vigilance purposes.
(d) Posts in newly established/temporary organisations (upto a period of 5 years from the date of establishment).
(e) Posts limited in number particularly in specialised fields where creation of a regular cadre is not feasible.
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.018 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.018 \, g}{18.018 \, g/mol} = 2 \, \text{mol}$$
- Moles to Moles:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 2 moles of $$H_2O$$.
- $$m = 2 \, \text{mol} \times 18.018 \, \text{g/mol} = 2 \, \text{mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{P_1} = \frac{P_1}{V_1}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – \Delta H_0$$
- Variables:
- $$\Delta H$$: Heat transferred at constant pressure.
- $$\Delta H_0$$: Heat transferred at constant pressure.
Hess’s Law (Hess’s Law)
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{reaction}} = \Delta H – \Delta H_0$$
- Variables:
- $$\Delta H$$: Heat transferred at constant pressure.
- $$\Delta H_0$$: Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$: Energy (K)
- $$E^\circ$$: Standard deviation (M)
- $$E$$: Standard deviation (M)
- $$E$$: Standard deviation (M)
- $$E$$: Standard deviation (M)
- $$n$$: Number of moles of electrons transferred
- $$F$$: Faraday constant (96,485 C/mol)
- $$Q$$: Reaction quotient
(1) The number of posts to be exempted may be decided in each CAB on a case to case basis.
The criteria for exemption of any particular category of posts from the “Rule of immediate absorption” should be non-availability of suitable persons for particular posts. All attempts should be made to fill up the post on a regular basis. The option for filling up of a post on deputation should be used as an exception when all other avenues have been exhausted.
- The procedure to be followed pursuant to these guidelines will be that each Autonomous body should seek exemption from the general rule of immediate absorption in respect of any particular post or posts or in respect of the organization as a whole, the proposal should be made by the Autonomous body to the administrative Ministry/Dept. concerned along with details for each post in proforma enclosed with this O.M. In the administrative Ministry the proposals may be scrutinized in accordance with these guidelines and only such proposals which have the approval of the Secretary of the administrative Ministry concerned may be referred to the Dept. of Pension & PW, indicating the full justification for granting such exemption. After a post or categories of posts is so exempted the Autonomous body/ Administrative Ministry concerned shall be competent to take decision in individual cases.
-
For the purpose of these orders, a Central Autonomous body is generally a non-profit making organization which is financed wholly or substantially from cess or Central Govt. grants. “Substantially” means that more than 50% of the expenditure of the Autonomous body is met through cess or Central Govt. grants. An Autonomous body may be a society registered under the ‘Societies Registration Act’, 1860 or a statutory body or a Central University having its own governing council whose memorandum of association/bye-laws etc., contain provision for complying with Govt. directives for carrying out its business in achieving the objectives for which the organization is established.
-
It has been observed by the Dept. of Pension & PW that sometimes proposals for exemption are received after the selection for the post had been made or without the approval of the Secretary of the concerned Ministry or at the fag end thus leaving no time for DOP&PW to examine the case properly. Therefore, proposals, complete in all respect and duly supported by the relevant documents may be furnished well in advance.
-
It has also been observed in the Dept. of Pension & PW that cadre authorities do not seek confirmation as to whether a particular post was exempted from the rule of immediate absorption in respect of their officers for cadre clearance before relieving them on deputation to Central autonomous bodies. In order to ensure compliance of the instructions, it has been decided to reiterate the existing instructions that all administrative Ministries/Departments
1. Introduction
1.1 Background
The study of quantum mechanics has revolutionized our understanding of the microscopic world. It provides a framework for describing the behavior of particles at atomic and subatomic scales. One of the key principles in quantum mechanics is the wave-particle duality, which states that particles can exhibit both wave-like and particle-like properties.
1.2 Objectives
The primary objective of this research is to explore the interaction between light and matter at the quantum level. Specifically, we aim to:
- Investigate the interaction between light and matter at the quantum level.
- Analyze the interaction between light and matter at the quantum level.
- Develop a quantum theory that describes the behavior of light at the quantum level.
2. Literature Review
2.1 Quantum Mechanics
Quantum mechanics is a fundamental theory in physics that provides a framework for describing the behavior of particles at atomic and subatomic scales. It provides a framework for describing the behavior of particles at atomic and subatomic scales. This research focuses on the analysis of quantum systems, including quantum systems with quantum entanglement and entanglement analysis.
2.2 Quantum Computing
Quantum computing leverages the principles of quantum mechanics to perform computations that are infeasible for classical computers. Quantum bits, or qubits, are the fundamental units of quantum information. Quantum bits are a fundamental concept in quantum mechanics, with their properties known to include their states in classical computers.
3. Methodology
3.1 Experimental Setup
The experimental setup involved a laser source with a laser source. The laser source was used to generate a quantum state, which was described by the lens source as a laser source. The laser source was used to generate a quantum state with entangled particles, which was described by the qubit source. The quantum state was measured using a laser source to generate a quantum state with entangled particles.
3.2 Data Collection
Data was collected over a period of three months. The data was analyzed using statistical methods, including the double-slit analysis and the double-slit analysis [1]. The data was analyzed using statistical methods and the qubit analysis [2] [3]. The results were compared with the experimental data to ensure accuracy.
4. Results and Discussion
4.1 Quantum Systems
The quantum systems were analyzed using the qubit analysis [4]. The qubit states were analyzed using statistical methods, including the double-slit analysis [5]. The results were compared with the classical qubit analysis [6]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| | |
4.2 Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [7]. The results were compared with the classical qubit analysis [8]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.3 Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [9]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.4 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [10]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.5 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [11]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.6 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [12]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.7 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [13]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.8 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [14]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
4.9 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [15]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.10 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [16]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.11 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [17]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.12 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [18]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.13 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [19]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.14 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [20]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.15 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [21]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.16 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [22]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.17 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [23]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
5.18 Quantum Computing in Quantum Computing
The quantum systems were analyzed using statistical methods and the double-slit analysis [24]. The results are summarized in the following table:
| Qubit | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
must get exemption from the rule of immediate absorption for appointment of Central Govt. servants on deputation basis to posts in Central Autonomous
under their administrative control. If a Central Govt. servant is allowed to
proceed to a Central autonomous body on deputation basis without obtaining
specific exemption for the post, the official will have to be treated as having
assigned from the Central Govt. and absorbed in the Central autonomous body.
He will then have to forego counting of past service for pensionary benefits in
the Central autonomous body. No ex-post-facto approval will be given.
- All cadre controlling authorities are, therefore, requested to get confirmation that the post for which an officer of the cadre has applied has been exempted from the Rule of immediate absorption before conveying cadre clearance to the concerned Ministry/Dept./Autonomous Body.
-
All Ministries/Depts. and all Central autonomous bodies under their administrative control, who decide to appoint serving Central Govt. servants on deputation basis to posts which are exempted from the Rule of immediate absorption should make a specific mention of the exemption in the vacancy circular/advertisement such a vacancy circular/advertisement is available to the candidate as well as the parent Ministry/Dept. for cadre clearance. It has also been decided that an authentic copy of the circular/advertisement clearly making a specific mention of communication conveying approval of the Dept. of Pension & PW for exemption may be enclosed with the proforma seeking cadre clearance to enable cadre controlling authority to examine to give cadre clearance expeditiously.
-
Period of deputation for Govt. employees against posts to be exempted shall be in accordance with the extant DOPT guidelines on the subject.
-
All administrative Ministries/Departments are requested to take note of the above decisions and also to bring the same to the notice of the Autonomous bodies under their administrative control for strict compliance by all concerned.
-
This issues with the approval of competent authority.
-
Hindi version will follow.
(Amitabh Dwivedi)
Under Secretary to the Government of India
Telephone No. 24644847
To
All Ministries/Departments. (As per mailing list)
97
–
4
ANNEXURE TO DOP&P W’S OM NO. 4/78/2006-P&P W (D) DATED OCTOBER 2007
PROFORMA
FURTHQUARIS TO BE FURNISHED BY THE CENTRAL AUTONOMOUS BODIES ETC. SEAL THE PURPOSE OF GRANT OF EXEMPTION FROM THE RULE OF IMMEDIATE ABSORPTION FROM THE DEPARTMENT OF PENSION & PENSIONERS’ WELFARE SO THAT SPECIFIC POSTS COULD BE FILLED UP BY TAKING CENTRAL GOVERNMENT EMPLOYEES ON DEPUTATION BASIS.
- Designation of the post
-
Pay scale
-
(i) Number of posts (sanctioned strength)/ and (ii) Number of posts proposed to be filled up on deputation
-
Brief description of functions of Posts proposed to be filled up in
-
Qualifications and experience prescribed in the notified Recruitment Rules (authentic copy of which may be attached) for appointment to the post.
-
In respect of each category of posts for which exemption is being sought, please give details about the hierarchy of posts below (their designation, pay scale, number of posts and method of recruitment) performing similar nature of duties. (This would help in examining if the posts can be filled by promotion from the feeder grades, within the organisation.)
-
Justification for filling the post on deputation, and not on immediate absorption basis.
-
(i) What is the prescribed period of deputation as per notified Recruitment Rules and (ii) Period for which exemption is being sought (i.e. the period after which it is proposed to revert to the normal procedure of appointment on immediate absorption basis.)
-
Date year of setting up of the Central Autonomous Body.
-
Details of previous exemption obtained, if any from this Dept. in respect of the post in proposal. Reference of Department of Pension & Pensioners’ Welfare must be quoted.
-
Whether the proposal has specific approval of Secretary of Administrative Ministry.
-
Name, designation and telephone number of the officer who could be invited for discussion in case of doubt clarification.
Note: This proforma is to be signed by an officer of the administrative Ministry not below the rank of Under Secretary.
25
.
ANNEXURE I
No AB-14017/2007-EST (RR)
Government of India
Ministry of Personnel, PG and Pensions
(Department of Personnel and Training)
New Delhi-110001
Feb. 29th 2008
OFFICE MEMORANDUM
Subject: Consolidated guidelines on deputation / foreign service for
members of the organized Group A and the Group B Services of
the Central Government
The issue regarding deputation/ foreign service to ex-cadre posts has
been reviewed and it has been decided that henceforth, the appointment for the
purpose of deputation / foreign service and provisions regulating tenure &
procedure of appointment would be according to guidelines enclosed.
2 For the time being, the guidelines contained in these instructions will
apply only to members of the organized Group ‘A and ‘B’ Services of the Central
Government. These orders will take effect from the date of issue and past cases
will not be reviewed based on these instructions. Hindi version will follow.
(Smita Kumar)
Director (E.3)
To
All Ministries/Departments of Government of India
Copy to :-
- The President’s Secretariat, New Delhi
- The Vice President’s Secretariat, New Delhi
- The Prime Minister’s Office, New Delhi, w i t : 1) No 10242/08/PMO/2008-
Pol dt 25.2 2008 - Cabinet Secretariat, New Delhi
- Rajya Sabha Secretariat/Lok Sabha Secretariat, New Delhi
- The Registrar General, Supreme Court of India
- The Registrar, Central Administrative Tribunal, Principal Bench, New Delhi
- The Comptroller and Auditor General of India, New Delhi
- Secretary, Union Public Service Commission, New Delhi
- Staff Selection Commission, New Delhi
89
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.015 \, \text{g/mol} = 18.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{V_1}{P_2V_2}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Equation: $$\Delta H = q_p + e^{\frac{-H_2p_0}{T}}$$
- Example: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
Hess’s Law
- Statement: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
- Example: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
Hess’s Law 2.0
- Statement: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- E: Cell potential
- R: Ideal gas constant
- F: Faraday constant
- Q: Reaction quotient
Acids and Bases
Arrhenius Theory
- Acid: Substance that dissociates in water to produce H⁺ ions.
- Base: Substance that dissociates in water to produce OH⁻ ions.
- Cathode: Substance that dissociates in water to produce OH⁻ ions.
Brønsted-Lowry Theory
- Acid: Proton donor.
- Base: Proton acceptor.
Lewis Theory
- Acid: Electron pair acceptor.
- Base: Electron pair donor.
Acids and Bases
Arrhenius Theory
- Acid: Proton acceptor.
- Base: Proton donor.
- Cathode: Proton donor.
Lewis Theory
- Acid: Electron pair donor.
- Base: Electron pair donor.
Brønsted-Lowry Theory
- Acid: Electron pair donor.
- Base: Electron pair donor.
Bases
Ideal Gas Law
- Equation: $$P_1V_1 = P_2V_2$$
- Statement: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
Lewis Theory
- Acid: Electron pair donor.
- Base: Electron pair donor.
Lewis Theory
- Acid: Electron pair donor.
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Statement: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
Hess’s Law
- Statement: The enthalpy change of a reaction is the same whether it occurs in one step or multiple steps.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Electrochemical Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- E: Cell potential
- R: Ideal gas constant
- F: Faraday constant
- Q: Reaction quotient
GUIDELINES FOR DEPUTATION / FOREIGN SERVICE OF CENTRAL GOVERNMENT OFFICERS
1.1 Central Staffing Scheme (CSS) :-
Posts that are to be covered:
– Ministries/Departments of Government of India
Procedure to be followed for appointment:
Civil Services Board (below JS), with ACC approval for JS and above
Tenure to be applicable:
– US level: 3 years
– DS level: 4 years
– Dir level: 5 years
– JS/AS level: 7 years (subject to 3 years in the second post, and any subject further to a minimum of 5 years in the Centre)
– AS level: 4 years
– Secy level: no ceiling.
1.2 Non-Central Staffing Scheme posts:-
1.2.1 Posts that are to be covered:
- Autonomous Institutions wholly or substantially funded or controlled by the Central Government
Procedure to be followed for appointment:
Search-cum-Selection Committee process as laid down in DoPT Office Memorandum No. 28/13/2006-FO/SM II (dated 07/2006 & 08/14/2006) NO AB 14047/11/2004-Esri(RR) (dated 30/7/05 OR as per approval) RRs OR as per statutory provisions for institutions covered by specific statutes (with ACC approval for Chief Executives carrying pay scales of Rs. 8400-27400 or above).
Tenure to be applicable: As provided under the Central Staffing Scheme.
1.2.2 Posts that are to be covered:
- CVGs
Procedure to be followed for appointment:
From DOPT panel with concurrence of CVC and Ministry concerned (with ACC approval for JS and above), i.e. as per current procedure.
Title: The Impact of Climate Change on Global Ecosystems
Introduction
Climate change is one of the most pressing environmental issues of our time. It affects ecosystems worldwide, leading to significant changes in biodiversity, habitat loss, and species extinction. This report explores the impacts of climate change on global ecosystems, focusing on key areas such as forests, oceans, and polar regions.
1. Forest Ecosystems
Forests play a crucial role in carbon sequestration and maintaining biodiversity. However, rising temperatures and changing precipitation patterns are altering forest ecosystems. Key impacts include:
- Increased frequency of wildfires: Rising temperatures and drought conditions have led to more frequent and severe wildfires, destroying vast areas of forests.
- Changes in species distribution: Shifts in temperature and precipitation patterns are altering forest ecosystems, disrupting ecosystem balance.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which are causing widespread wildfires.
2. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea temperatures and drought conditions have led to more frequent and severe wildfires, disrupting ecosystem balance.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, disrupting ecosystem balance, and species extinction.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, disrupting ecosystem balance, and species extinction.
3. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea temperatures and reduced CO₂ levels are altering ocean currents, disrupting ecosystem balance.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, disrupting ecosystem balance, and species extinction.
4. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea temperatures and reduced CO₂ levels are altering ocean currents, disrupting ecosystem balance.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, disrupting ecosystem balance, and species extinction.
5. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: The Arctic is warming at twice the rate of the global average, leading to sea ice loss.
- Glacial retreat: Melting glaciers and their presence in the Arctic are rising, affecting ocean currents, impacting marine ecosystems.
- Glacial retreat: Melting glaciers and their presence in the Arctic are altering ocean currents, disrupting ecosystem balance, and species extinction.
Conclusion
Climate change poses a significant threat to global ecosystems, with far-reaching consequences for biodiversity and human societies. By understanding the impacts of climate change on global ecosystems, we can help you reduce and mitigate the impacts of climate change.
References
- IPCC (Intergovernmental Panel on Climate Change). (2021). Climate Change 2021: The Physical Science Basis.
- WWF (World Wildlife Fund). (2020). Living Planet Report 2020.
- NASA Global Climate Change. (2022). Vital Signs: Global Temperature.
Tenure to be applicable:
Maximum of 5 years. (A deputation of 3 + 4 years is permissible when no officer moves from one PSI to another)
1.2.3 Posts that are to be covered:
Central PSUs or PSUs of another State or PSUs wholly or substantially owned and controlled by two or more States
Procedure to be followed for appointment:
Subject to exemption from Immediate Absorption Rule (AACR), PESU Search Committee (with ACC approval for JS and above)
Tenure to be applicable: As provided under the Central Staffing Scheme
1.2.4 Posts that are to be covered:
Constitutional Bodies or staff officers of Heads of Constitutional Bodies
Procedure to be followed for appointment:
Civil Services Board QR on request by name, subject to signature clearance and suitability (with ACC approval for JS and above)
Tenure to be applicable: As provided under the Central Staffing Scheme
1.2.5 Posts that are to be covered:
Statutory Bodies set up by an Act of Parliament or staff officers of Heads of such Statutory Bodies
Procedure to be followed for appointment:
For appointments below JS level – through a Committee under the Chairmanship of Secretary (Personnel) with the approval of MOS(PP)
For appointments of JS and above level – through CSR with the approval of ACC
Tenure to be applicable: As provided under the Central Staffing Scheme
1.2.6 Posts that are to be covered:
Non-permanent, Non-Statutory Bodies with a specific term set up through executive orders/notification by the Central Government Administrative Reforms Commissions, Pay Commission, National Manufacturing Competitiveness Commission, Sachar Committee, and the Commissions etc.
Procedure to be followed for appointment:
Civil Services Board (with ACC approval for JS and above)
Tenure to be applicable: As provided under the Central Staffing Scheme
Title: The Impact of Climate Change on Global Ecosystems
Introduction
Climate change is one of the most pressing environmental issues of our time. It affects ecosystems worldwide, leading to significant changes in biodiversity, habitat loss, and species extinction. This report explores the impacts of climate change on global ecosystems, focusing on key areas such as forests, oceans, and polar regions.
1. Forest Ecosystems
Forests play a crucial role in carbon sequestration and maintaining biodiversity. However, rising temperatures and changing precipitation patterns are altering forest ecosystems. Key impacts include:
- Increased frequency of wildfires: Rising temperatures and drought conditions have led to more frequent and severe wildfires, destroying vast areas of forests.
- Changes in species distribution: Shifts in temperature and precipitation patterns are altering species distribution, leading to species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which are causing widespread wildfires.
2. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea temperatures and drought conditions have led to more frequent and severe wildfires, threatening species like polar bears and seals.
- Changes in ocean currents: Altered ocean currents are causing widespread sea-level rise, threatening species like polar bears and seals.
- Changes in ocean currents: Shifts in ocean currents are altering ocean currents, threatening species like polar bears and seals.
3. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: The Arctic is warming at twice the rate of the global average, leading to sea ice loss.
- Glacial retreat: Melting glaciers and their presence in the Arctic are altering the ocean currents, threatening species like polar bears and seals.
- Permafrost thawing: Thawing permafrost releases stored carbon and methane, further accelerating global warming.
4. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: Melting glaciers and their presence in the Arctic are altering sea ice and their presence in the Arctic are altering ocean currents.
- Glacial retreat: Melting glaciers and their presence in the Arctic are altering ocean currents, threatening species like polar bears and seals.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, threatening species like polar bears and seals.
5. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: Melting glaciers and their presence in the Arctic are altering sea ice and their presence in the Arctic are altering ocean currents.
- Glacial retreat: Melting glaciers and their presence in the Arctic are altering ocean currents, threatening species like polar bears and seals.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, threatening species like polar bears and seals.
Conclusion
Climate change poses a significant threat to global ecosystems, with far-reaching consequences for biodiversity and human societies. By understanding the impacts of climate change on global ecosystems, we can help you reduce and preserve the natural world and ensure the long-term health and well-being of your planet.
References
- IPCC (Intergovernmental Panel on Climate Change). (2021). Climate Change 2021: The Physical Science Basis.
- WWF (World Wildlife Fund). (2020). Living Planet Report 2020.
- NASA. (2021). Vital Signs: Global Temperature.
Provided that, if an officer moves from a CSS to a non-CSS post, or vice versa, she/he shall be eligible for an additional tenure of two years, subject to at least two years on either post.
1.3 Ex-Cadre Deputation.
Posts that are to be covered:
To another post in Central Government: State Government where Recruitment rules / regulations etc exist and deputation is one of the methods of appointment.
Procedure to be followed for appointment:
With the concurrence of the cadre controlling authority, borrowing Department and with the approval of the authority competent for filling in the post.
Tenure to be applicable:
According to the provisions of DOPT QM no. 2/29/91-Estt.(Pay-II) dated the 5th January 1994 as amended from time to time.
2.1 International Organizations.
Posts that are to be covered:
i) UN Organizations.
ii) International Financial Institutions like World Bank, IMF, ADB, etc.
iii) Multilateral organizations of which India is a member like I.M.A.WTO.
– Commonwealth Organization, International Fund of India, SAARC etc.
iv) Bilateral Bodies set up under the Vienna Convention, i.e. Embassies and Bodies set up under them, like USAID, DFID, NORAD, etc.
v) International NGOs or Funding Organizations from which India receives technical/financial assistance like International Red Cross Society, Action Aid, Aga Khan Foundation, Fort Foundation, etc.
Procedure to be followed for appointment:
A Committee under the Chairmanship of Cabinet Secretary with Secretary (Personnel), Finance Secretary will screen all proposals for deputation on foreign service terms of officers of the level of JS and above on a case to case basis, after the proposals have been approved by the Cabinet Controlling Authority. Such screening in the case of officers below the level of JS will be by a Committee chaired by the Secretary of the Cabinet Controlling Ministry/Department with a member each, not below the level of JS from the DOPT and Department of Expenditure. A final decision on the recommendations of the Screening Committee may be taken at the level of Minister-in-charge in the case of officers holding posts below JS-level and with the approval of PM in the case of officers holding JS-level posts above.
Title: The Impact of Climate Change on Global Ecosystems
Introduction
Climate change is one of the most pressing environmental issues of our time. It affects ecosystems worldwide, leading to significant changes in biodiversity, habitat loss, and species extinction. This report explores the impacts of climate change on global ecosystems, focusing on key areas such as forests, oceans, and polar regions.
1. Forest Ecosystems
Forests play a crucial role in carbon sequestration and maintaining biodiversity. However, climate change is causing significant changes in ecosystems, leading to significant changes in biodiversity. Key impacts include:
- Increased frequency of wildfires: Rising temperatures and drought conditions have led to more frequent and severe wildfires, destroying vast areas of forests.
- Changes in species distribution: Shifts in temperature and precipitation patterns are altering species distribution, leading to species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which cause widespread tree mortality.
2. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea levels and drought conditions have led to more frequent and severe wildfires, destroying vast areas of oceans.
- Changes in ocean currents: Altered ocean currents affect nutrient distribution, leading to increased ocean temperatures.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which cause widespread tree mortality.
3. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea levels and drought conditions have led to more frequent and severe wildfires, destroying vast areas of oceans.
- Changes in ocean currents: Altered ocean currents are altering species distribution, leading to species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which cause widespread tree mortality.
4. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: The Arctic is warming at twice the rate of the global average, leading to sea ice loss.
- Glacial retreat: Melting glaciers and their presence in the Arctic are rising, threatening coastal ecosystems.
- Permafrost thawing: Thawing permafrost releases stored carbon and methane, further accelerating global warming.
5. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea levels and drought conditions have led to more frequent wildfires, destroying vast areas of oceans.
- Changes in ocean currents: Altered ocean currents are altering species distribution, affecting species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which cause widespread tree mortality.
Conclusion
Climate change poses significant threats to global ecosystems, with far-reaching consequences for biodiversity and human societies. By understanding the impacts of climate change on global ecosystems, we can help you reduce and mitigate the impacts of climate change.
References
- IPCC (Intergovernmental Panel on Climate Change). (2021). Climate Change 2021: The Physical Science Basis.
- WWF (World Wildlife Fund). (2020). Living Planet Report 2020.
- NASA Global Climate Change. (2022). Vital Signs: Global Temperature.
Provided that for appointment to posts listed at (iv), the Foreign Secretary or the MEA Secretary concerned shall also be a member of the Committee under the chairmanship of Cabinet Secretary for officers of IS and above level. For officers below IS level, a nominee of Foreign Secretary, not below the level of IS would be included in the screening committee.
Provided further that for appointments to posts listed at (v) the concurrence of MHA shall be taken.
Tenure to be applicable:
Maximum of 5 years at a stretch
2.2 Autonomous body, trust, society, etc. not controlled by the Government, or a private body.
Posts that are to be covered:
(i) Registered Societies or Trusts or Foundations or non-organizations or NGOs or cooperatives;
(ii) Apex bodies of Industries and Commerce.
Provided that such autonomous or private bodies fulfill all four of the following criteria:
(a) they are functionally autonomous of the Central and State Governments;
(b) they are not substantially funded by the Central and State Governments;
(c) the Central or State Governments do not have powers to give their directions; and
(d) they are not companies registered under the Registration Companies Act.
Procedure to be followed for appointment:
As in Para 2.1
Tenure to be applicable:
Maximum of 5 years at a stretch
2.3 Terms and conditions for deputation: foreign service not covered under this OMI would be as per DOPT OM No. 2/29/91-Ext (Pay-11) dated the 5th January 1991 amended from time to time. For the foreign service, terms and conditions in which amend the guidelines of 1994 to the extent they are at variance.
2.4 The total period of foreign service under Para 2.1 and 2.2 above shall not exceed maximum of 7 years in the entire service.
2.5 There shall be a mandatory “Cooling Off” requirement after every period of deputation and foreign service. The length of “Cooling Off”
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.018 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.018 \, g}{18.018 \, g/mol} = 2 \, \text{mol}$$
- Moles to Moles:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.018 \, g/m^{3} = 2 \, \text{mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{P_1} = \frac{P_1}{V_1}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p + \Delta H_0$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$: Energy (K)
- $$E^\circ$$: Heat transferred (J)
- $$E$$: Heat transferred (K)
- $$Q$$: Reaction quotient
shall be as follows:
(i) For JS level (Rs. 18400-22400/) and below -3 years
(ii) For AS level (Rs. 22400 – 2500/) – 1 years
(iii) For Secretary level- nil
2.6 The Consolidated Deputation/Foreign service guidelines for organized Gr. ‘A’ & ‘B’ services shall come into force with prospective effect.
2.8 Appointments for which orders have already been issued shall not be affected by these guidelines
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.015 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.015 \, \text{g}}{18.015 \, \text{g/mol}} = 18.015 \, \text{g/mol}$$
- Moles to Mass:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.015 \, \text{g/mol} = 18.015 \, \text{g/mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{V_1}{P_2V_2}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Equation: $$\Delta H = q_p + e^{\frac{-H_2p_0}{T}}$$
- Example: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Example: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
Hess’s Law 2.0
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- E: Cell potential
- R: Ideal gas constant
- F: Faraday constant
- Q: Reaction quotient
Acids and Bases
Arrhenius Theory
- Acid: Substance that dissociates in water to produce H⁺ ions.
- Base: Substance that dissociates in water to produce OH⁻ ions.
- Cathode: Substance that dissociates in water to produce OH⁻ ions.
Brønsted-Lowry Theory
- Acid: Proton donor.
- Base: Proton acceptor.
Lewis Theory
- Acid: Electron pair acceptor.
- Base: Electron pair donor.
Nuclear Chemistry
Radioactive Decay
- Alpha Decay (α): Emission of a radioactive substance.
- Beta Decay (β): Emission of a radioactive substance.
Half-Life (t₁/₂)
- Definition: The time required for a quantity to decay to half of its original value.
- Equation: $$t₁/₂ = (ln(t₁/₂)) / (ln(t₂))$$
Organic Chemistry
Functional Groups
- Alkanes: -C=O -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C=18 -C
Annexure-1
Terms And Conditions For Foreign Service
- The general principle of public interest shall be the overriding factor in deciding foreign service under this rule. The competent authority shall also see whether there is any enrichment of the experience of the officer by such deputation.
-
Officers who are on foreign service would run the disqualification of not being considered for empanelment under the Central Staffing Scheme during the currency of their foreign service and also if they earn ACRs on return to their cadre.
-
ICRA clearance shall be required for foreign service to an organization receiving foreign donations.
-
All such cases of foreign service shall be considered only with the consent of the officer concerned and the approval of the cadre controlling authority.
-
The foreign service will not be considered under any circumstances as a mandatory posting.
-
A Central Government Officer shall be eligible for foreign service only after he has completed 5 years of service.
-
A Central Government Officer shall be eligible for foreign service only if he is clear from vigilance angle.
-
For foreign service, the officer should not have dealt with the borrowing organization in the last five years.
-
An officer shall not be allowed to proceed on foreign service to organizations in which he or any of his blood relations is connected with the setting up/management of the organization.
-
Mode of selection for the post may be based on advertisement, nomination or direct offer.
-
The limit of 5 years in one stretch and 7 years in the entire career for foreign service to organizations covered under Para 2.1 and 2.2 shall not be extended under any circumstances. The officer shall be deemed to have resigned from service in case he/she fails to join the Government within one month of completion of his/her approved tenure with the concerned organization.
Title: The Impact of Climate Change on Global Ecosystems
Introduction
Climate change is one of the most pressing environmental issues of our time. It affects ecosystems worldwide, leading to significant changes in biodiversity, habitat loss, and species extinction. This report explores the impacts of climate change on global ecosystems, focusing on key areas such as forests, oceans, and polar regions.
1. Forest Ecosystems
Forests play a crucial role in carbon sequestration and maintaining biodiversity. However, rising temperatures and changing precipitation patterns are altering forest ecosystems. Key impacts include:
- Increased frequency of wildfires: Rising temperatures and drought conditions have led to more frequent and severe wildfires, destroying vast areas of forests.
- Changes in species distribution: Shifts in temperature and precipitation patterns are altering species distribution, leading to species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which are causing widespread wildfires.
2. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Rising sea temperatures and drought conditions have led to more frequent wildfires, destroying vast areas of oceans.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, threatening species survival and biodiversity.
3. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The impacts include:
- Increased frequency of wildfires: Rising sea temperatures and reduced CO₂ levels are altering the marine environment, leading to sea-level rise and loss of marine life.
- Changes in ocean currents: Altered ocean currents are altering ocean currents, threatening species survival and biodiversity.
4. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: The Arctic is warming at twice the rate of the global average, leading to sea ice loss.
- Glacial retreat: Melting glaciers and their presence in the Arctic are altering the ocean currents, threatening species survival and biodiversity.
5. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: Melting glaciers and their presence in the Arctic are altering sea ice and threatening species survival and biodiversity.
- Glacial retreat: Melting glaciers and their presence in the Arctic are altering sea ice and threatening species survival and biodiversity.
Conclusion
Climate change poses a significant threat to global ecosystems, with far-reaching consequences for biodiversity and human societies. By reducing greenhouse gas emissions and reducing greenhouse gas emissions, we can protect our planet for future generations.
References
- IPCC (Intergovernmental Panel on Climate Change). (2021). Climate Change 2021: The Physical Science Basis.
- WWF (World Wildlife Fund). (2020). Living Planet Report 2020.
- NASA Global Climate Change. (2022). Vital Signs: Global Temperature.
- While serving in Constitutional statutory, multilateral or bilateral organization, international financial organizations, the officer shall be eligible to draw pay and allowances as per the scheme of the borrowing organization. In the other organizations, the officer may opt for his grade pay, or the pay of the post, whichever is more beneficial to him.
-
While on foreign service, the service conditions of the officer shall continue to be regulated under the relevant Service Rules. Other terms and conditions may be in accordance with standard terms devised from time to time.
-
The provisions of paying to the Government 1/3rd of the amount of fee earned by the officer during short-term assignments with international organizations may also be removed. There will not be any distinction between international organizations and other national organizations in this respect.
-
Participation in the pension scheme – The officers on foreign service, except to Constitutional bodies, which may have their own regular pension schemes, shall not be permitted to join the pension schemes of the organization under any circumstances. A Central Government Officer may join the Pension scheme of the UN bodies in accordance with the relevant rules. On joining the same, the service rendered by the officer during the deputation period shall not be counted as qualifying for pension.
-
The entire expenditure in respect of pension and leave salary contribution for the period of foreign service shall be borne by the borrowing organization, falling which by the officer. However, those allowed joining the pension schemes of the organizations mentioned above shall not be required to make pension contributions.
-
Performance appraisal /ACRs during the period of foreign service. The competent authority in the organization accepting the officer shall provide an ACR/Performance appraisal written in such form as prescribed under rules.
-
The terms and conditions of foreign service shall be finalised by the concerned administrative Ministry/Department, in accordance with the standard terms and conditions prescribed by the DOFT.
-
Notwithstanding anything above, the Government shall have the absolute right to refuse permission or recall an officer from foreign service.
-
An officer on foreign service shall be considered for promotion on his turn.
Title: The Impact of Climate Change on Global Ecosystems
Introduction
Climate change is one of the most pressing environmental issues of our time. It affects ecosystems worldwide, leading to significant changes in biodiversity, habitat loss, and species extinction. This report explores the impacts of climate change on global ecosystems, focusing on key areas such as forests, oceans, and polar regions.
1. Forest Ecosystems
Forests play a crucial role in carbon sequestration and maintaining biodiversity. However, climate change is causing significant changes in ecosystems, leading to significant changes in biodiversity. Key impacts include:
- Increased frequency of wildfires: Rising temperatures and drought conditions have led to more frequent and severe wildfires, destroying vast areas of forests.
- Changes in species distribution: Shifts in temperature and precipitation patterns are altering species distribution, leading to species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which cause widespread tree mortality.
2. Ocean Ecosystems
Oceans absorb a significant portion of the excess heat and carbon dioxide (CO₂) produced by human activities. The consequences include:
- Increased frequency of wildfires: Warmer temperatures have increased the survival rates of wildfires, disrupting marine life, leading to widespread tree mortality.
- Changes in species distribution: Shifts in temperature and precipitation patterns are altering species distribution, leading to species extinction.
- Insect outbreaks: Warmer temperatures have increased the survival rates of pests like bark beetles, which cause widespread tree mortality.
3. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: The Arctic is warming at twice the rate of the global average, leading to sea ice loss.
- Glacial retreat: Melting glaciers and their potential for foodborne activity are causing sea ice loss.
- Permafrost thawing: Thawing permafrost releases stored carbon and methane, further accelerating global warming.
4. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: Melting glaciers and their potential for foodborne activity are causing sea ice loss.
- Glacial retreat: Melting glaciers and their potential for foodborne activity are causing sea ice loss.
- Permafrost thawing: Thawing permafrost releases stored carbon and methane, further accelerating global warming.
5. Polar Ecosystems
Polar regions are particularly vulnerable to climate change due to their sensitivity to temperature changes. Key impacts include:
- Melting of sea ice: Melting glaciers and their potential for foodborne activity are causing sea ice loss.
- Glacial retreat: Melting glaciers and their potential for foodborne activity are causing sea ice loss.
- Permafrost thawing: Thawing permafrost releases stored carbon and methane, further accelerating global warming.
Conclusion
Climate change poses significant threats to global ecosystems, with far-reaching consequences for biodiversity and human societies. By understanding the impacts of climate change on global ecosystems, we can help you reduce the risk of human activities and ensure the sustainability of your planet.
References
- IPCC (Intergovernmental Panel on Climate Change). (2021). Climate Change 2021: The Physical Science Basis.
- WWF (World Wildlife Fund). (2020). Living Planet Report 2020.
- NASA. (2021). Vital Signs: Global Temperature.
Grade-wise status of stagnation in CSS
Assistant to SO Grade: The stagnation in the grade of Assistant was acute as Assistants of SL 1992 have been included in the SO SL 2008. Select Lists of SOs are in arrears. If updated Select Lists of SOs are brought out, there would be gradual improvement and on an average, Assistants from seniority quota are likely to be included in the SO SL in 10 years.
| Assistant SL | Inclusion in SO SL Year (likely) |
|---|---|
| 1992(part) | 2008 |
| 1993(part) | 2009 |
| 1994 | 2010 |
| 1995-97 (part) | 2011 |
| 1997-2002 | 2012 |
| 2003 | 2013 and 2014 |
| 2004 | 2014 |
| 2005 | 2015 |
| 2006 | 2016 |
| 2007 | 2017 |
| 2008 | 2018 |
With the increase in the strength of DS/US/SO, all the vacancies will trickle down to Assistant grade which will reduce the time taken for promotion from Assistant to SO grade by 1-2 years. Hence there will not be any significant stagnation in the Assistant grade.
SO to US Grade: Part of SOs of select list 2003 have been included in USSL 2011. However, since SOSL 2003 and 2004 are larger, all these SOs are likely to be
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.018 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.018 \, g}{18.018 \, g/mol} = 2 \, \text{mol}$$
- Moles to Moles:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.018 \, g/m^{3} = 2 \, \text{mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{P_1} = \frac{P_1}{V_1}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H = q_p + \Delta H_0$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$: Energy (K)
- $$E^\circ$$: Heat transferred (J)
- $$E$$: Heat transferred (K)
- $$Q$$: Reaction quotient
covered in USSL 2012, 2013 and 2014. Subsequent SO SLs are comparatively smaller and likely time taken for their inclusion in the US Select Lists would be around 10 years.
| SOSL | Likely Inclusion in USSL (for
Genl. Category) | Years taken/to be taken (likely) |
| — | — | — |
| 2003 | 2011,2012 and 2013 | 8 to 10 years |
| 2004 | 2014,2015 | 10-11 years |
| 2005 | 2015 | 10 |
| 2006 | 2016 | 10 |
| 2007 | 2017 | 10 |
| 2008 | 2018 | 10 |
With the increase in the strength of DS/US, there will be more vacancies available which will further reduce the time taken for promotion from SO to US grade by another 1-2 years and hence there will be practically no stagnation in promotion from SO to US grade.
US to DS grade: There has been much delay in bringing out the regular Select Lists of US also. US SL 2003 was brought out in August, 2009 and US 2004-08 in January, 2010 prior to which many officers were working in ad hoc capacity for a long time. On an average, it took 13-15 years for SOs to be included in the US SLs 2003-08. The US SL 2003 is large and more than 800 SOs of Select Lists 1984 to 1990 (part) were included in it. It has taken about 5 years to promote the entire USs of SL 2003 to the grade of DS. While Regular Select Lists of DS are pending due to litigation, the tentative projection shows some improvement initially but later US SLs are larger and hence it will take more time for the USs to get included in the DS SLs.
| US SL Year | Inclusion year and size DS SL year wise
(likely) | Years to be taken |
| — | — | — |
| 2003 | $2013(95)^{a}$ | 10 |
| $2004-2005$ | $2014(103)^{a}$ | 10 |
Chemistry
Chemical Reactions
Balancing Chemical Equations
- Write the unbalanced equation:
- Example: $$C_3H_8 + O_2 \rightarrow CO_2 + H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
- Balance the equation:
- Example: $$2C_3H_8 + 7O_2 \rightarrow 6CO_2 + 8H_2O$$
Types of Reactions
- Combination Reaction:
- Example: $$2H_2 + O_2 \rightarrow 2H_2O$$
- Decomposition Reaction:
- Example: $$2H_2O_2 \rightarrow 2H_2O + O_2$$
- Single Displacement Reaction:
- Example: $$Zn + 2HCl \rightarrow ZnCl_2 + H_2$$
- Double Displacement Reaction:
- Example: $$AgNO_3 + NaCl \rightarrow AgCl + NaNO_3$$
- Combustion Reaction:
- Example: $$CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O$$
Stoichiometry
Mole Concept
- Mole (mol): The amount of substance containing as many particles (atoms, molecules, ions) as there are atoms in exactly 12 grams of carbon-12.
- Avogadro’s Number: $$6.022 \times 10^{23}$$ particles per mole.
Molar Mass
- Molar Mass: The mass of one mole of a substance.
- Example: The molar mass of water ($$H_2O$$) is 18.018 g/mol.
Calculations
- Moles to Mass:
- Formula: $$n = \frac{m}{M}$$
- Example: Calculate the number of moles of $$H_2O$$ in 18 grams of water.
- $$n = \frac{18.018 \, g}{18.018 \, g/mol} = 2 \, \text{mol}$$
- Moles to Moles:
- Formula: $$m = n \times M$$
- Example: Calculate the mass of 1 mole of $$H_2O$$.
- $$m = 18.018 \, g / 18.018 \, g/mol = 2 \, \text{mol}$$
Gas Laws
Ideal Gas Law
- Equation: $$PV = nRT$$
- Variables:
- $$P$$: Pressure (atm)
- $$V$$: Volume (L)
- $$n$$: Number of moles (mol)
- $$R$$: Ideal gas constant (0.0821 L·atm/mol·K)
- $$T$$: Temperature (K)
Boyle’s Law
- Equation: $$P_1V_1 = P_2V_2$$
- Variables:
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
- P₂: Volume (L)
- P₃: Pressure (atm)
- P₁: Pressure (atm)
Boyle’s Law (Boyle’s Law)
- Equation: $$\frac{P_1V_1}{P_2V_2} = \frac{P_2V_2}{T_1} = \frac{P_1}{T_2}$$
Thermochemistry
Enthalpy (H)
- Definition: The heat content of a system at constant pressure.
- Equation: $$\Delta H = q_p$$
- Variables:
- $$q_p$$: Heat transferred at constant pressure.
- $$q_p$$: Heat transferred at constant pressure.
Hess’s Law
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{rest}} = \Delta H – Q_{\text{rest}}$$
- Variables:
- $$\Delta H$$: Heat transferred at constant pressure.
- $$\Delta H$$: Heat transferred at constant pressure.
Hess’s Law (Hess’s Law)
- Statement: The enthalpy change for a reaction is the same whether it occurs in one step or multiple steps.
- Equation: $$\Delta H_{\text{rest}} = \Delta H – Q_{\text{rest}}$$
- Variables:
- $$H$$: Heat transferred at constant pressure.
- $$Q_{\text{rest}}$$: Heat transferred at constant pressure.
Electrochemistry
Oxidation and Reduction
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
Galvanic Cells
- Definition: A cell that converts chemical energy into electrical energy.
- Components:
- Anode: Oxidation occurs.
- Cathode: Reduction occurs.
- Salt Bridge: Connects the two half-cells.
Nernst Equation
- Equation: $$E = E^\circ – \frac{RT}{nF} \ln Q$$
- Variables:
- $$E$$: Energy (K)
- $$E^\circ$$: Standard deviation of the energy (J)
- $$E$$: Standard deviation of the energy (J)
- $$T$$: Temperature (K)
- $$n$$: Number of electrons transferred
- $$F$$: Faraday constant (96,485 C/mol)
- $$Q$$: Reaction quotient
| 2006 | $2015(80)^{*}$ | 09 |
|---|---|---|
| $2006-07$ | $2016(76)^{*}$ | 10 |
| 2007 | $2017(85)^{*}$ | 10 |
| 2008 | $2018(91)^{*}$ | 10 |
| 2009 (size 268) | $2019(95)^{*}$ | 10 |
| $2020(103)^{*}$ | 11 | |
| 2010 (size 215) | $2021-2022$ | $11-12$ |
| 2011 (size 371) | $2023-2024$ | $12-13$ |
With the increase of 75 posts DS, the time taken for promotion from US to DS grade is likely to get reduced by a maximum up to 1 year.
DS to Director
There is no stagnation as all eligible DS with the residency of 5 years have been promoted to the grade of Director.
.